| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232509 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 10 Pages |
•Chalcones with electron donor acceptor group has been studied.•The absorption and emission spectrum were sensitive to solvent polarity.•Fluorescence quantum yield highly depend on polarity of solvents.•Both chalcones show fluorescence quenching by silver nanoparticles.
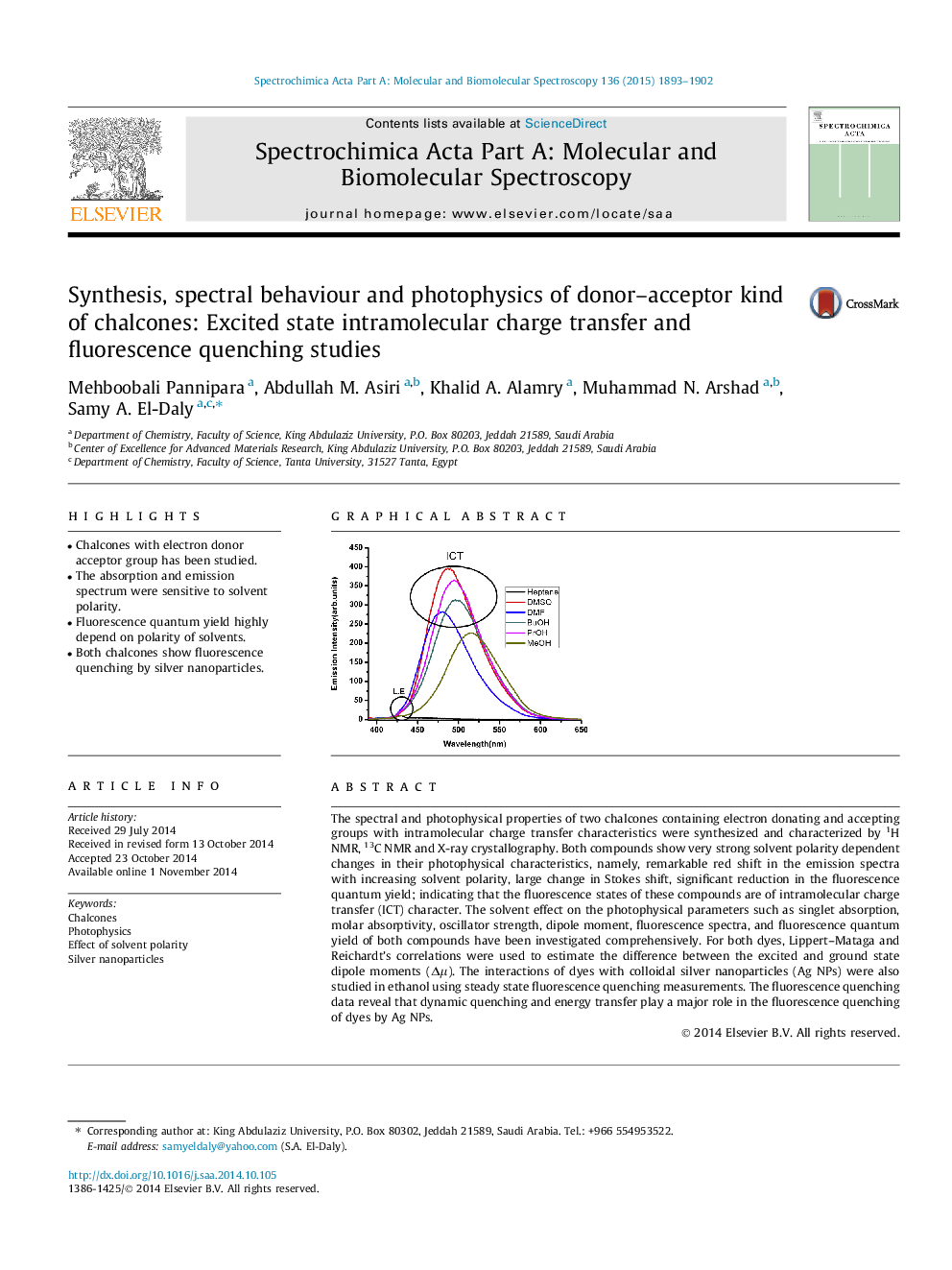
The spectral and photophysical properties of two chalcones containing electron donating and accepting groups with intramolecular charge transfer characteristics were synthesized and characterized by 1H NMR, 13C NMR and X-ray crystallography. Both compounds show very strong solvent polarity dependent changes in their photophysical characteristics, namely, remarkable red shift in the emission spectra with increasing solvent polarity, large change in Stokes shift, significant reduction in the fluorescence quantum yield; indicating that the fluorescence states of these compounds are of intramolecular charge transfer (ICT) character. The solvent effect on the photophysical parameters such as singlet absorption, molar absorptivity, oscillator strength, dipole moment, fluorescence spectra, and fluorescence quantum yield of both compounds have been investigated comprehensively. For both dyes, Lippert–Mataga and Reichardt’s correlations were used to estimate the difference between the excited and ground state dipole moments (Δμ). The interactions of dyes with colloidal silver nanoparticles (Ag NPs) were also studied in ethanol using steady state fluorescence quenching measurements. The fluorescence quenching data reveal that dynamic quenching and energy transfer play a major role in the fluorescence quenching of dyes by Ag NPs.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide