| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232513 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 9 Pages |
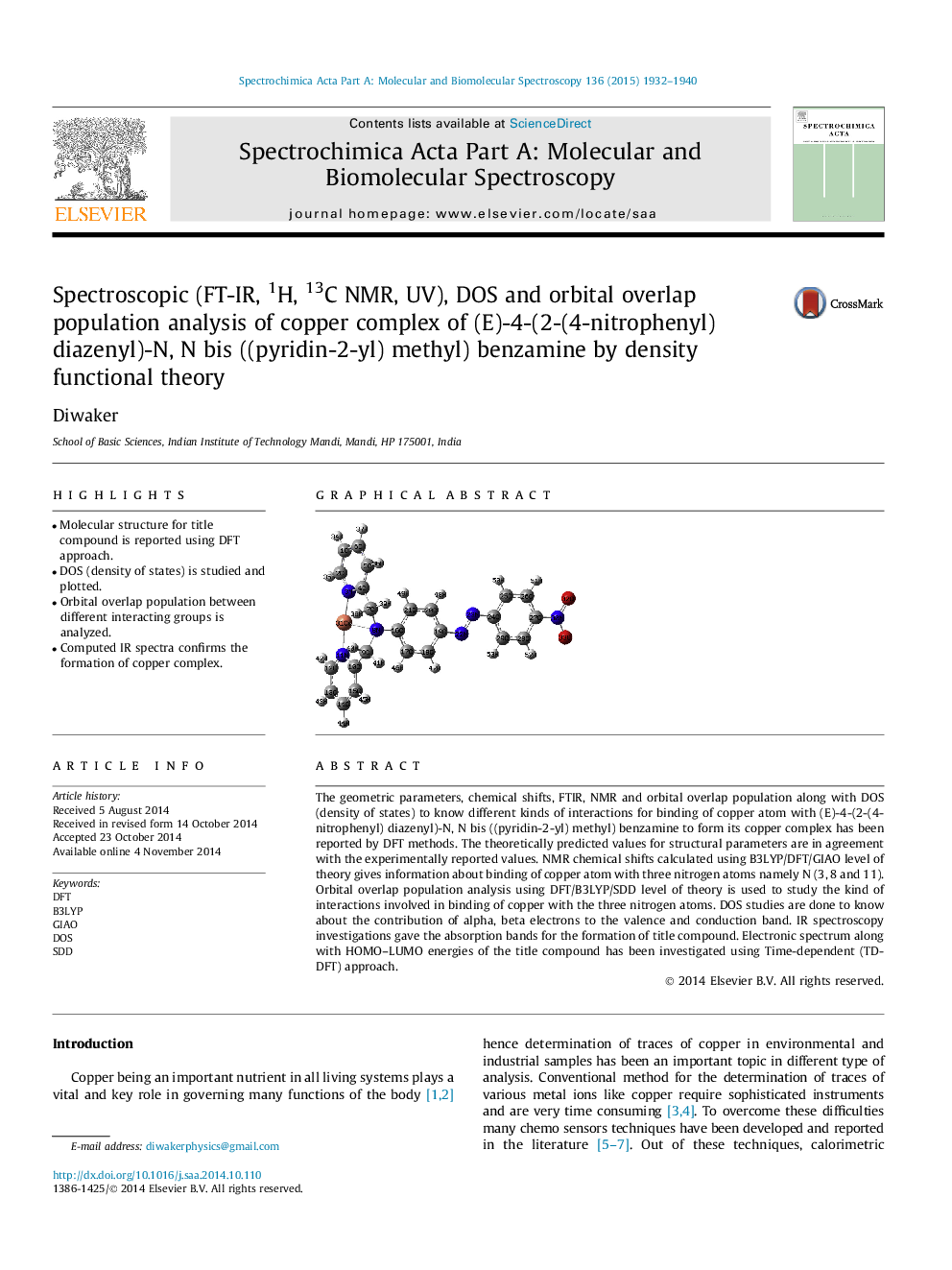
•Molecular structure for title compound is reported using DFT approach.•DOS (density of states) is studied and plotted.•Orbital overlap population between different interacting groups is analyzed.•Computed IR spectra confirms the formation of copper complex.
The geometric parameters, chemical shifts, FTIR, NMR and orbital overlap population along with DOS (density of states) to know different kinds of interactions for binding of copper atom with (E)-4-(2-(4-nitrophenyl) diazenyl)-N, N bis ((pyridin-2-yl) methyl) benzamine to form its copper complex has been reported by DFT methods. The theoretically predicted values for structural parameters are in agreement with the experimentally reported values. NMR chemical shifts calculated using B3LYP/DFT/GIAO level of theory gives information about binding of copper atom with three nitrogen atoms namely N (3, 8 and 11). Orbital overlap population analysis using DFT/B3LYP/SDD level of theory is used to study the kind of interactions involved in binding of copper with the three nitrogen atoms. DOS studies are done to know about the contribution of alpha, beta electrons to the valence and conduction band. IR spectroscopy investigations gave the absorption bands for the formation of title compound. Electronic spectrum along with HOMO–LUMO energies of the title compound has been investigated using Time-dependent (TD-DFT) approach.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide