| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232524 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 7 Pages |
•Solvatochromic study of π → π* absorption band of azo-benzene derivatives.•Spectral data correlated with energetic characteristics obtained by HyperChem.•Excited state dipole moments and polarizabilities of the studied molecules.•Comparison between the interaction energy in solute–solvent molecular pairs.
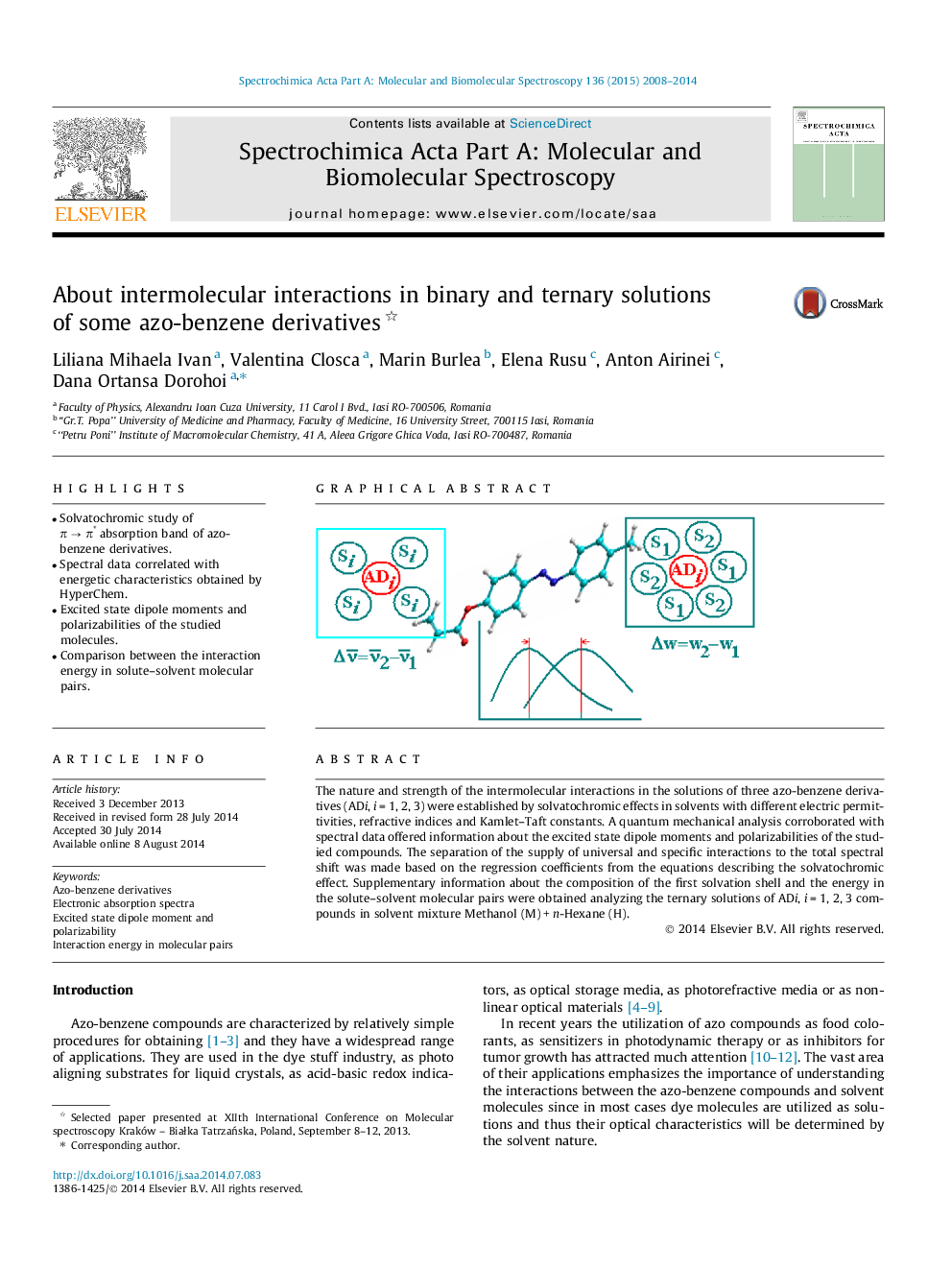
The nature and strength of the intermolecular interactions in the solutions of three azo-benzene derivatives (ADi, i = 1, 2, 3) were established by solvatochromic effects in solvents with different electric permittivities, refractive indices and Kamlet–Taft constants. A quantum mechanical analysis corroborated with spectral data offered information about the excited state dipole moments and polarizabilities of the studied compounds. The separation of the supply of universal and specific interactions to the total spectral shift was made based on the regression coefficients from the equations describing the solvatochromic effect. Supplementary information about the composition of the first solvation shell and the energy in the solute–solvent molecular pairs were obtained analyzing the ternary solutions of ADi, i = 1, 2, 3 compounds in solvent mixture Methanol (M) + n-Hexane (H).
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide