| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232674 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 7 Pages |
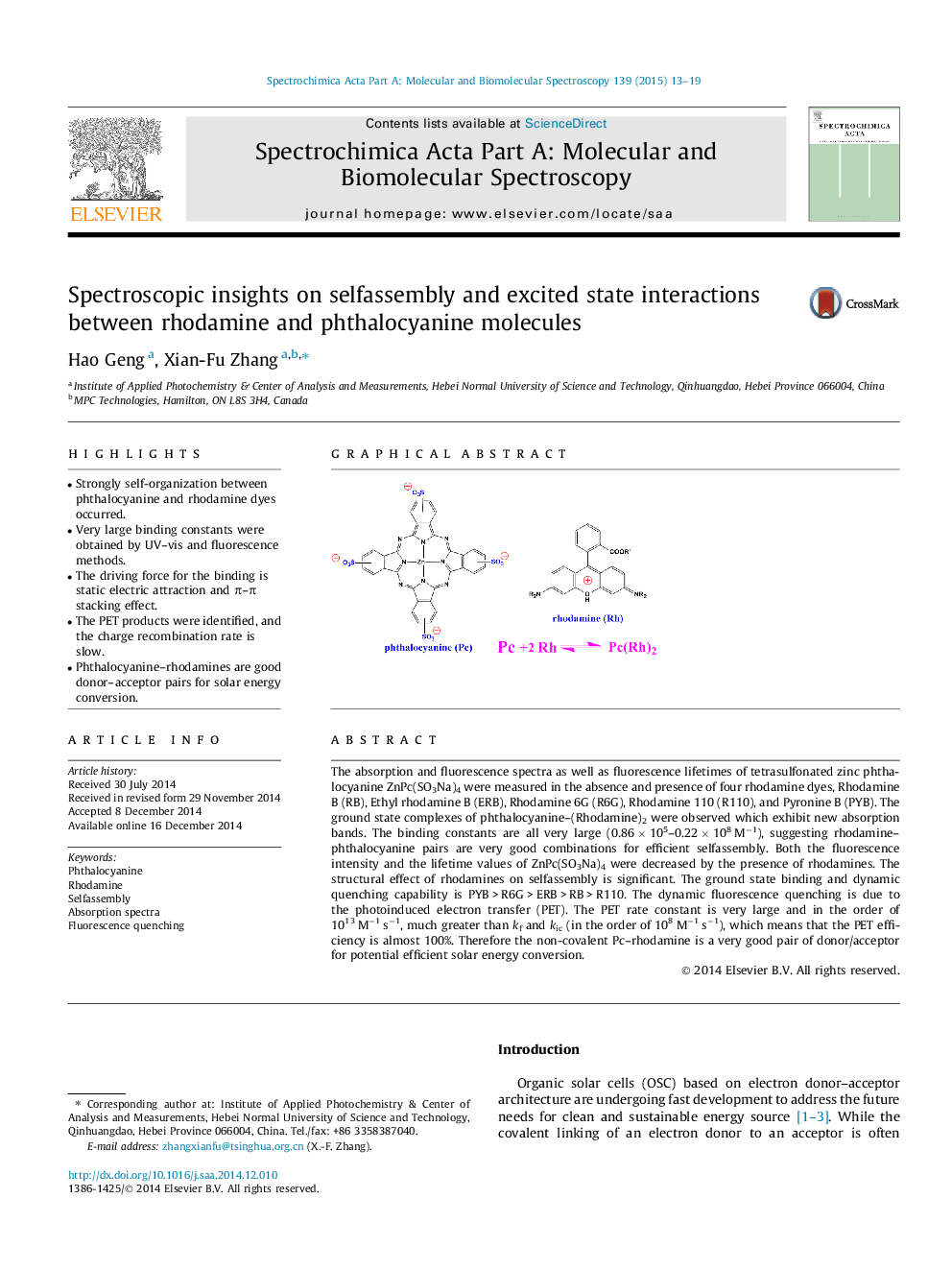
•Strongly self-organization between phthalocyanine and rhodamine dyes occurred.•Very large binding constants were obtained by UV–vis and fluorescence methods.•The driving force for the binding is static electric attraction and π–π stacking effect.•The PET products were identified, and the charge recombination rate is slow.•Phthalocyanine–rhodamines are good donor–acceptor pairs for solar energy conversion.
The absorption and fluorescence spectra as well as fluorescence lifetimes of tetrasulfonated zinc phthalocyanine ZnPc(SO3Na)4 were measured in the absence and presence of four rhodamine dyes, Rhodamine B (RB), Ethyl rhodamine B (ERB), Rhodamine 6G (R6G), Rhodamine 110 (R110), and Pyronine B (PYB). The ground state complexes of phthalocyanine–(Rhodamine)2 were observed which exhibit new absorption bands. The binding constants are all very large (0.86 × 105–0.22 × 108 M−1), suggesting rhodamine–phthalocyanine pairs are very good combinations for efficient selfassembly. Both the fluorescence intensity and the lifetime values of ZnPc(SO3Na)4 were decreased by the presence of rhodamines. The structural effect of rhodamines on selfassembly is significant. The ground state binding and dynamic quenching capability is PYB > R6G > ERB > RB > R110. The dynamic fluorescence quenching is due to the photoinduced electron transfer (PET). The PET rate constant is very large and in the order of 1013 M−1 s−1, much greater than kf and kic (in the order of 108 M−1 s−1), which means that the PET efficiency is almost 100%. Therefore the non-covalent Pc–rhodamine is a very good pair of donor/acceptor for potential efficient solar energy conversion.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide