| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232679 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 5 Pages |
•DFT/TDDFT method has been performed to study the ESIPT.•The nature of excited-state hydrogen bond dynamic has been investigated.•ESIPT of 8-hydroxyquinoline is facilitated by strengthening of the electronic excited-state hydrogen bond.•The radiationless deactivation via internal conversion is demonstrated.
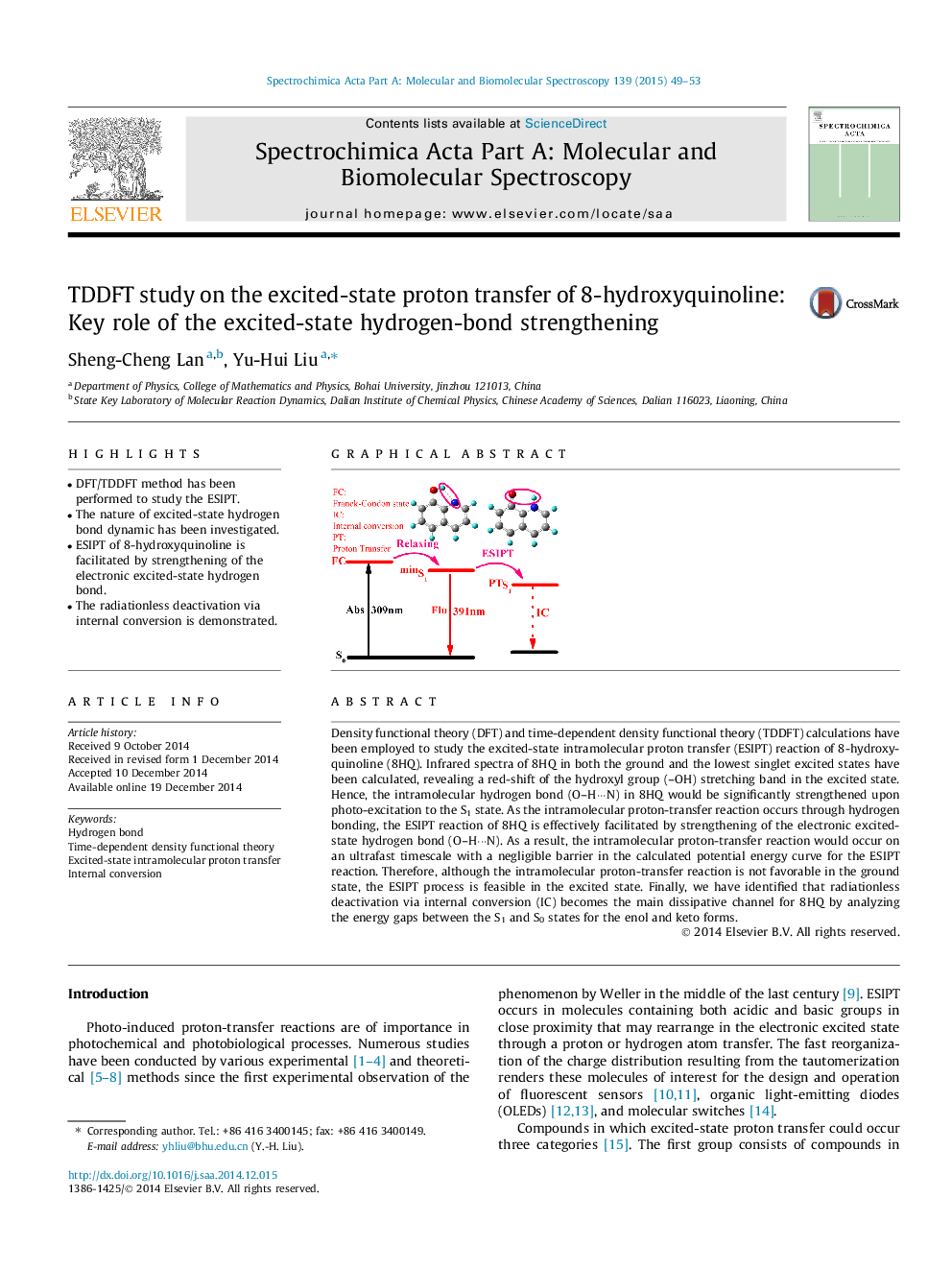
Density functional theory (DFT) and time-dependent density functional theory (TDDFT) calculations have been employed to study the excited-state intramolecular proton transfer (ESIPT) reaction of 8-hydroxyquinoline (8HQ). Infrared spectra of 8HQ in both the ground and the lowest singlet excited states have been calculated, revealing a red-shift of the hydroxyl group (–OH) stretching band in the excited state. Hence, the intramolecular hydrogen bond (O–H···N) in 8HQ would be significantly strengthened upon photo-excitation to the S1 state. As the intramolecular proton-transfer reaction occurs through hydrogen bonding, the ESIPT reaction of 8HQ is effectively facilitated by strengthening of the electronic excited-state hydrogen bond (O–H···N). As a result, the intramolecular proton-transfer reaction would occur on an ultrafast timescale with a negligible barrier in the calculated potential energy curve for the ESIPT reaction. Therefore, although the intramolecular proton-transfer reaction is not favorable in the ground state, the ESIPT process is feasible in the excited state. Finally, we have identified that radiationless deactivation via internal conversion (IC) becomes the main dissipative channel for 8HQ by analyzing the energy gaps between the S1 and S0 states for the enol and keto forms.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide