| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232690 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 8 Pages |
•[Cu(en)2]2+ has been immobilized onto the montmorillonite-K10 and montmorillonite-KSF.•The prepared catalyst showed great activities towards the decolorization of Chromotrope 2R.•The reaction kinetics of color removal confirmed the formation of a brown intermediate which interacts with the dye molecules.
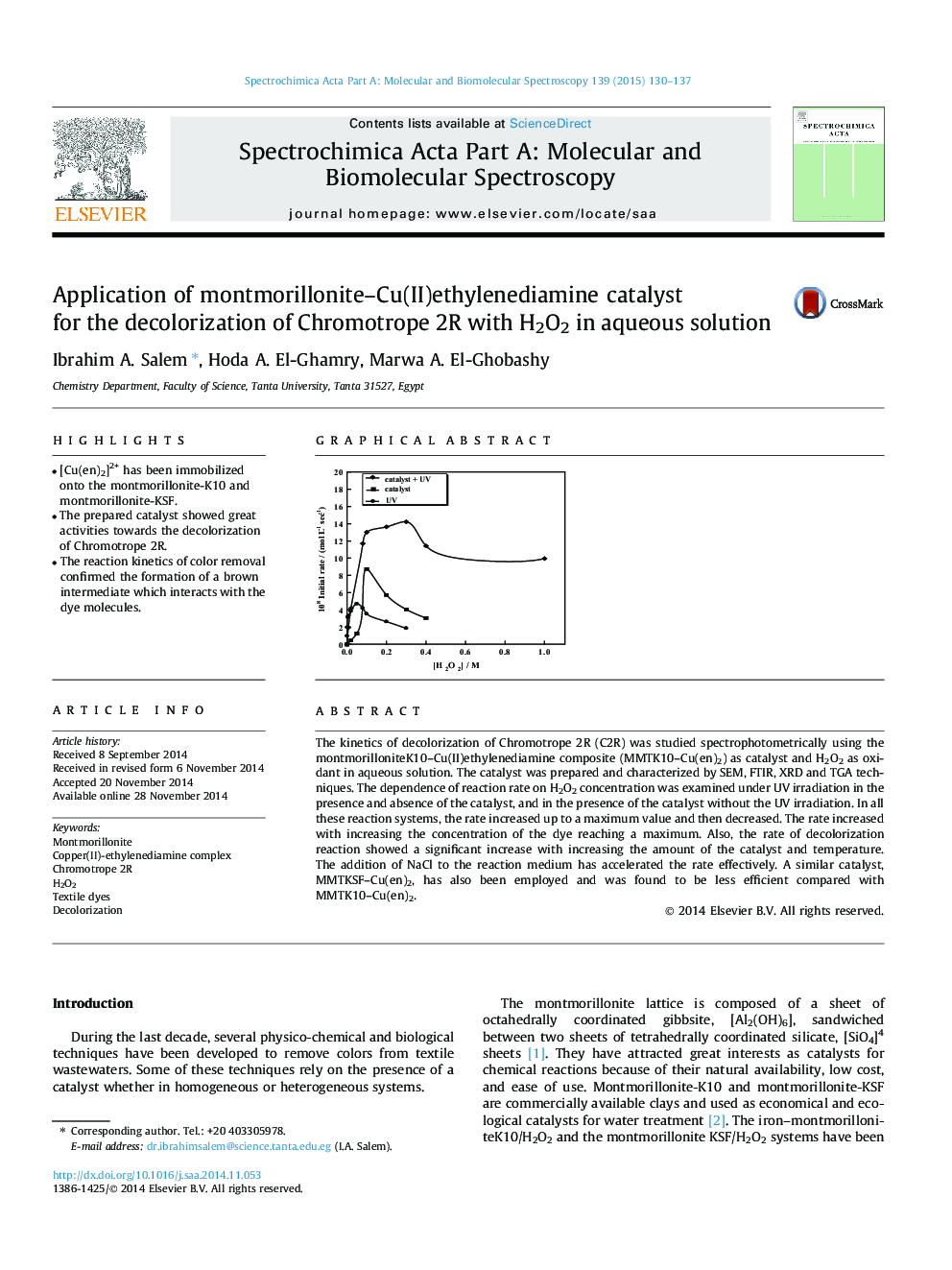
The kinetics of decolorization of Chromotrope 2R (C2R) was studied spectrophotometrically using the montmorilloniteK10–Cu(II)ethylenediamine composite (MMTK10–Cu(en)2) as catalyst and H2O2 as oxidant in aqueous solution. The catalyst was prepared and characterized by SEM, FTIR, XRD and TGA techniques. The dependence of reaction rate on H2O2 concentration was examined under UV irradiation in the presence and absence of the catalyst, and in the presence of the catalyst without the UV irradiation. In all these reaction systems, the rate increased up to a maximum value and then decreased. The rate increased with increasing the concentration of the dye reaching a maximum. Also, the rate of decolorization reaction showed a significant increase with increasing the amount of the catalyst and temperature. The addition of NaCl to the reaction medium has accelerated the rate effectively. A similar catalyst, MMTKSF–Cu(en)2, has also been employed and was found to be less efficient compared with MMTK10–Cu(en)2.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide