| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232709 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 4 Pages |
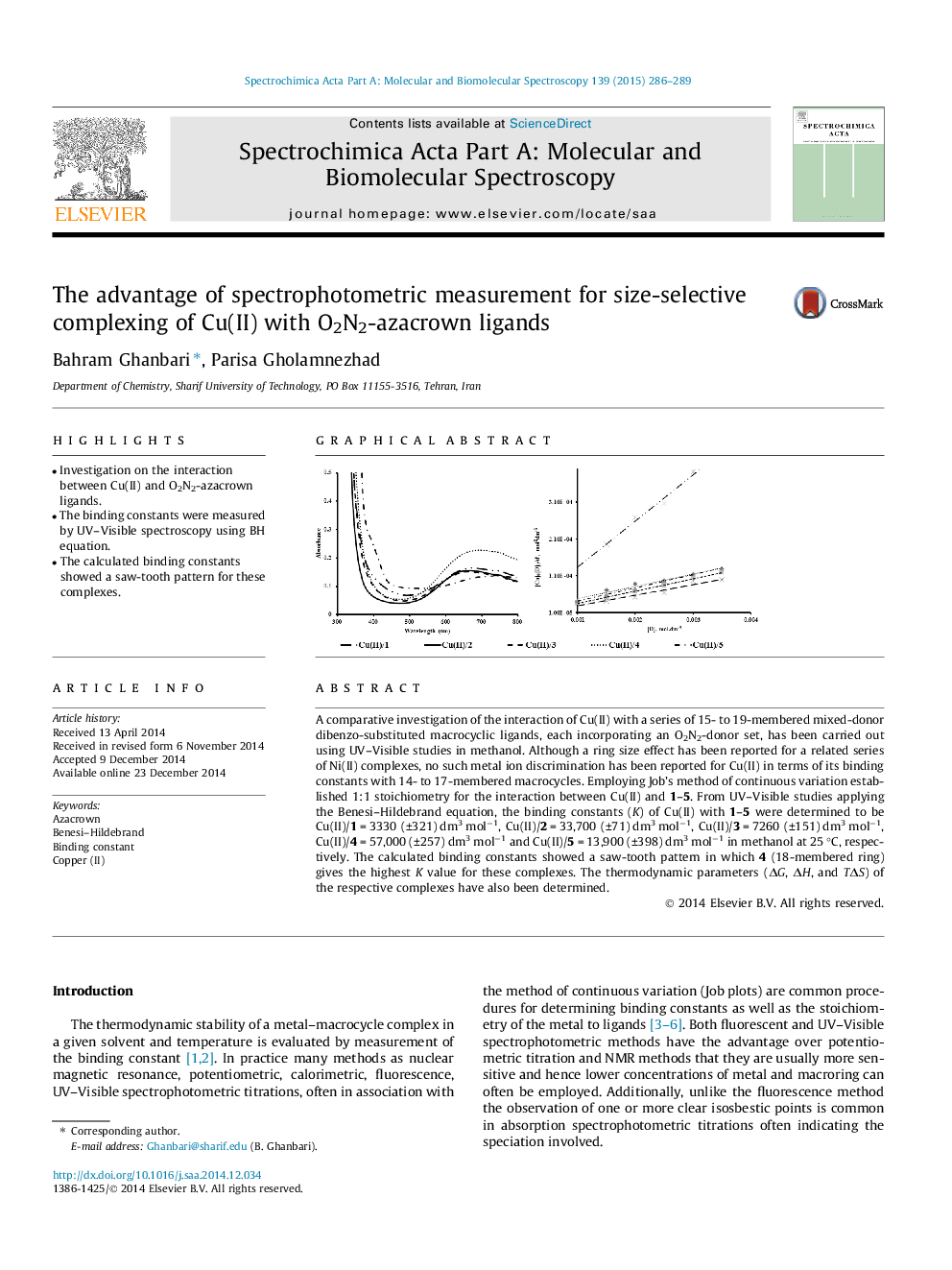
•Investigation on the interaction between Cu(II) and O2N2-azacrown ligands.•The binding constants were measured by UV–Visible spectroscopy using BH equation.•The calculated binding constants showed a saw-tooth pattern for these complexes.
A comparative investigation of the interaction of Cu(II) with a series of 15- to 19-membered mixed-donor dibenzo-substituted macrocyclic ligands, each incorporating an O2N2-donor set, has been carried out using UV–Visible studies in methanol. Although a ring size effect has been reported for a related series of Ni(II) complexes, no such metal ion discrimination has been reported for Cu(II) in terms of its binding constants with 14- to 17-membered macrocycles. Employing Job’s method of continuous variation established 1:1 stoichiometry for the interaction between Cu(II) and 1–5. From UV–Visible studies applying the Benesi–Hildebrand equation, the binding constants (K) of Cu(II) with 1–5 were determined to be Cu(II)/1 = 3330 (±321) dm3 mol−1, Cu(II)/2 = 33,700 (±71) dm3 mol−1, Cu(II)/3 = 7260 (±151) dm3 mol−1, Cu(II)/4 = 57,000 (±257) dm3 mol−1 and Cu(II)/5 = 13,900 (±398) dm3 mol−1 in methanol at 25 °C, respectively. The calculated binding constants showed a saw-tooth pattern in which 4 (18-membered ring) gives the highest K value for these complexes. The thermodynamic parameters (ΔG, ΔH, and TΔS) of the respective complexes have also been determined.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide