| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232711 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 6 Pages |
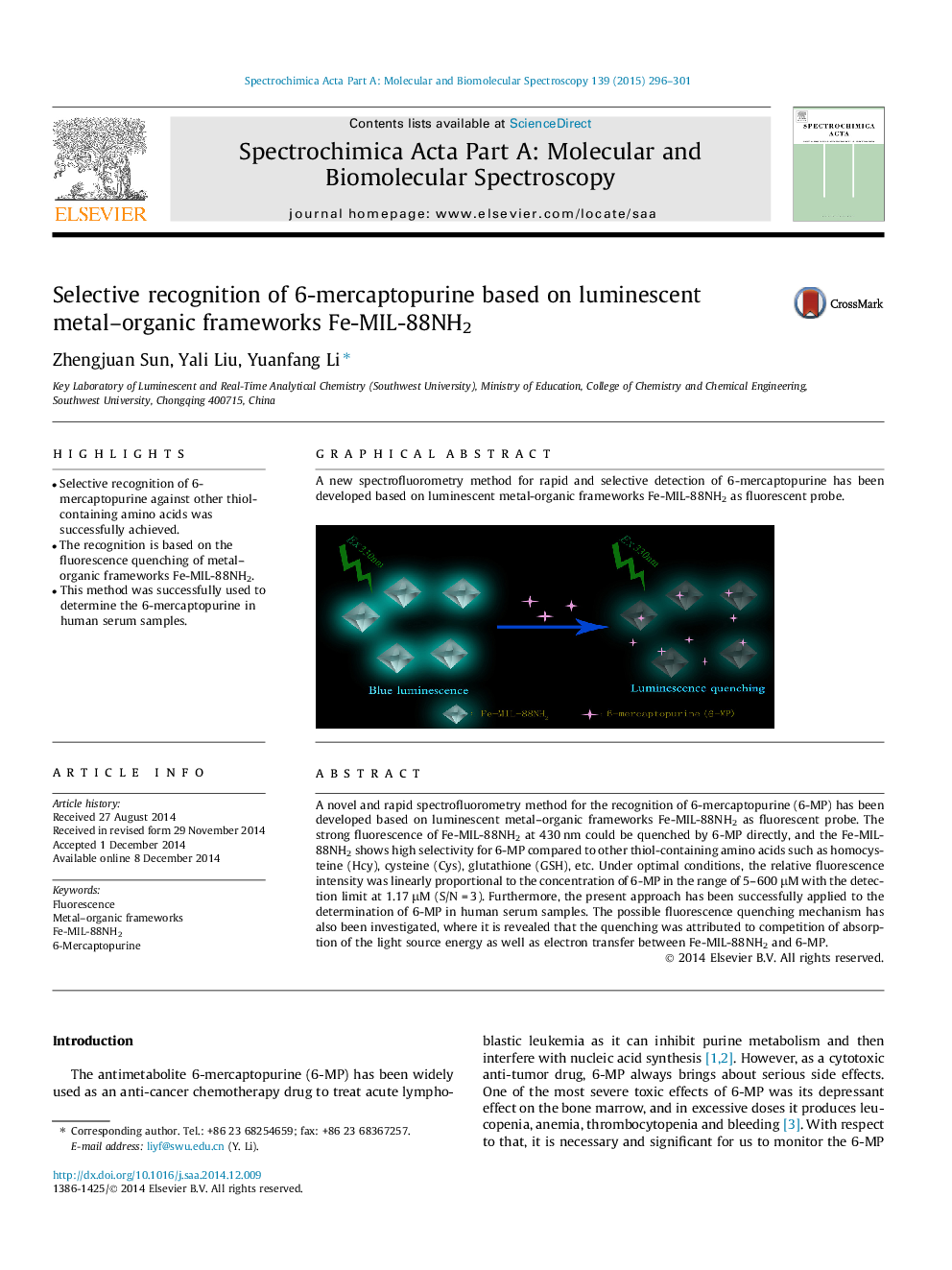
•Selective recognition of 6-mercaptopurine against other thiol-containing amino acids was successfully achieved.•The recognition is based on the fluorescence quenching of metal–organic frameworks Fe-MIL-88NH2.•This method was successfully used to determine the 6-mercaptopurine in human serum samples.
A novel and rapid spectrofluorometry method for the recognition of 6-mercaptopurine (6-MP) has been developed based on luminescent metal–organic frameworks Fe-MIL-88NH2 as fluorescent probe. The strong fluorescence of Fe-MIL-88NH2 at 430 nm could be quenched by 6-MP directly, and the Fe-MIL-88NH2 shows high selectivity for 6-MP compared to other thiol-containing amino acids such as homocysteine (Hcy), cysteine (Cys), glutathione (GSH), etc. Under optimal conditions, the relative fluorescence intensity was linearly proportional to the concentration of 6-MP in the range of 5–600 μM with the detection limit at 1.17 μM (S/N = 3). Furthermore, the present approach has been successfully applied to the determination of 6-MP in human serum samples. The possible fluorescence quenching mechanism has also been investigated, where it is revealed that the quenching was attributed to competition of absorption of the light source energy as well as electron transfer between Fe-MIL-88NH2 and 6-MP.
Graphical abstractA new spectrofluorometry method for rapid and selective detection of 6-mercaptopurine has been developed based on luminescent metal-organic frameworks Fe-MIL-88NH2 as fluorescent probe.Figure optionsDownload full-size imageDownload as PowerPoint slide