| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232718 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 4 Pages |
•We expanded the traditional electrolysis method.•Electrolysis experiments can be performed below 100 °C.•Potassium alum crystals are colored electrolytically for the first time.•SO3−, SO2− and O3− hole-trapped centers are produced in colored crystals.•Defect centers are mainly from thermal-electric decomposition of SO42− radicals.
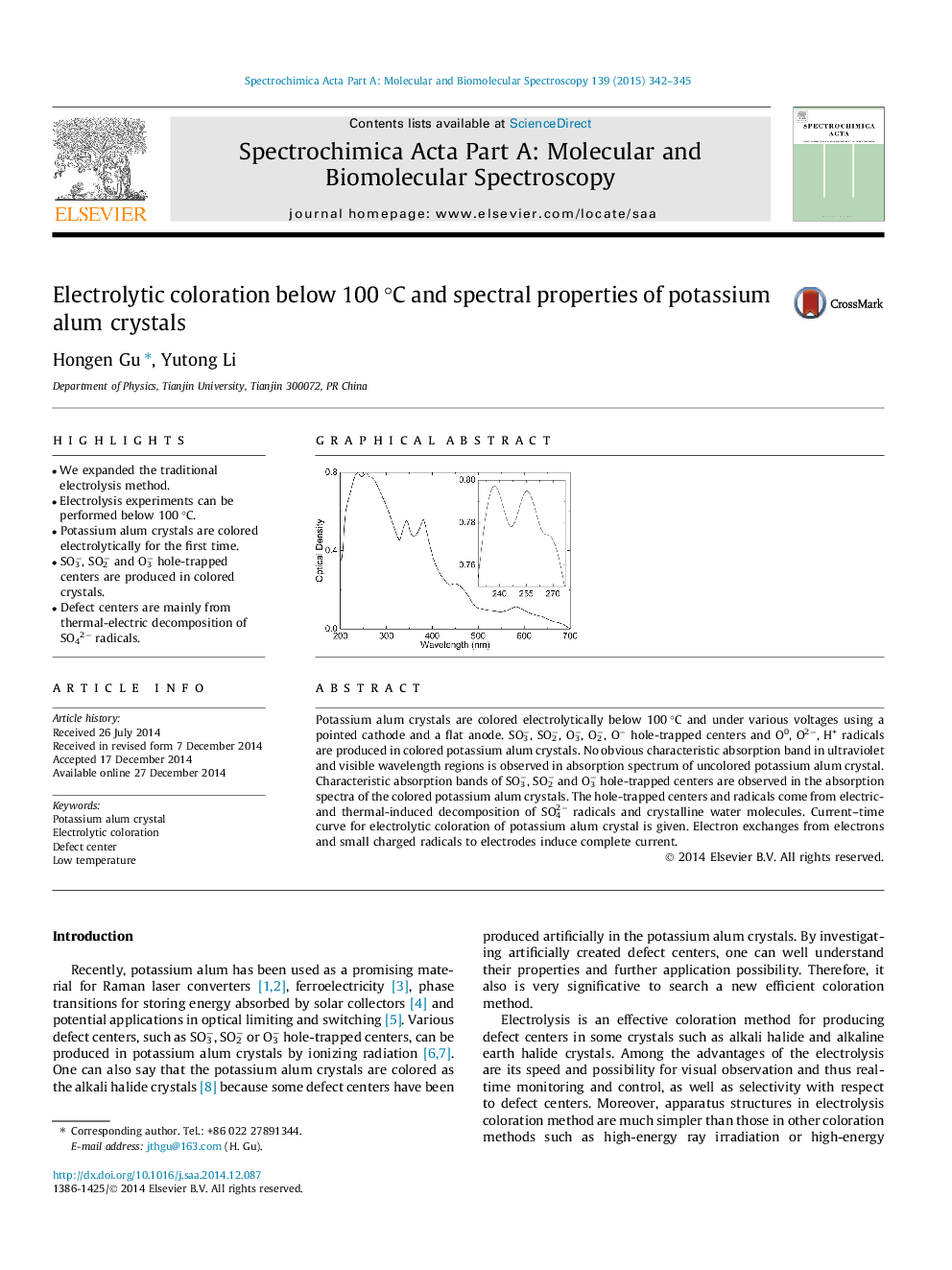
Potassium alum crystals are colored electrolytically below 100 °C and under various voltages using a pointed cathode and a flat anode. SO3−, SO2−, O3−, O2−, O− hole-trapped centers and O0, O2−, H+ radicals are produced in colored potassium alum crystals. No obvious characteristic absorption band in ultraviolet and visible wavelength regions is observed in absorption spectrum of uncolored potassium alum crystal. Characteristic absorption bands of SO3−, SO2− and O3− hole-trapped centers are observed in the absorption spectra of the colored potassium alum crystals. The hole-trapped centers and radicals come from electric- and thermal-induced decomposition of SO42− radicals and crystalline water molecules. Current–time curve for electrolytic coloration of potassium alum crystal is given. Electron exchanges from electrons and small charged radicals to electrodes induce complete current.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide