| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232830 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 7 Pages |
•The polarized IR spectra of two 3PhPz polymorphs were quantitatively analyzed.•We explained the origin of the temperature effects in the spectra.•The H/D isotopic self-organization effects also were recognized.•HB anti-co-operativity differentiates the IR spectra of two systems.
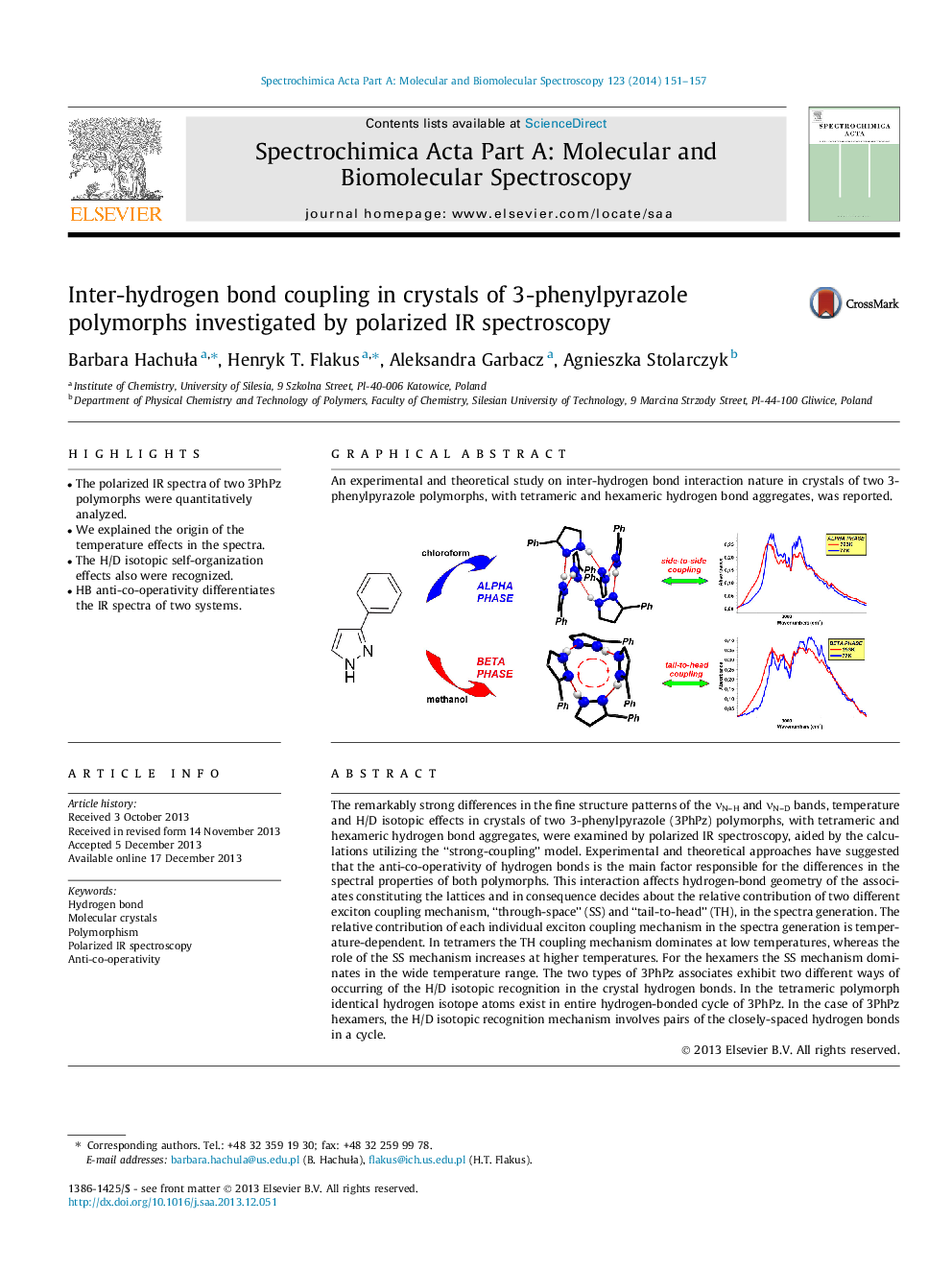
The remarkably strong differences in the fine structure patterns of the νN–H and νN–D bands, temperature and H/D isotopic effects in crystals of two 3-phenylpyrazole (3PhPz) polymorphs, with tetrameric and hexameric hydrogen bond aggregates, were examined by polarized IR spectroscopy, aided by the calculations utilizing the “strong-coupling” model. Experimental and theoretical approaches have suggested that the anti-co-operativity of hydrogen bonds is the main factor responsible for the differences in the spectral properties of both polymorphs. This interaction affects hydrogen-bond geometry of the associates constituting the lattices and in consequence decides about the relative contribution of two different exciton coupling mechanism, “through-space” (SS) and “tail-to-head” (TH), in the spectra generation. The relative contribution of each individual exciton coupling mechanism in the spectra generation is temperature-dependent. In tetramers the TH coupling mechanism dominates at low temperatures, whereas the role of the SS mechanism increases at higher temperatures. For the hexamers the SS mechanism dominates in the wide temperature range. The two types of 3PhPz associates exhibit two different ways of occurring of the H/D isotopic recognition in the crystal hydrogen bonds. In the tetrameric polymorph identical hydrogen isotope atoms exist in entire hydrogen-bonded cycle of 3PhPz. In the case of 3PhPz hexamers, the H/D isotopic recognition mechanism involves pairs of the closely-spaced hydrogen bonds in a cycle.
Graphical abstractAn experimental and theoretical study on inter-hydrogen bond interaction nature in crystals of two 3-phenylpyrazole polymorphs, with tetrameric and hexameric hydrogen bond aggregates, was reported.Figure optionsDownload full-size imageDownload as PowerPoint slide