| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232833 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 11 Pages |
•The interactions between HHb and five alkaloids have been investigated.•Hydrophobic and electrostatic interactions play major role in the binding process.•The influence of molecular structure on the binding aspects has been investigated.•Molecular docking was also applied in the binding study.
This work studied the interaction of human hemoglobin (HHb) with aminophylline, acefylline, caffeine, theophylline and diprophylline systematically by UV–vis absorption spectroscopy and fluorescence spectroscopy in combination with molecular modeling. Five alkaloids caused the fluorescence quenching of HHb by the formation of alkaloids-HHb complex. The binding constants and thermodynamic parameters were obtained. The hydrophobic and electrostatic interactions were the predominant intermolecular forces to stabilize these complexes. Results of thermodynamic analysis and molecular modeling showed that aminophylline was the strongest quencher and diprophylline was the weakest quencher.
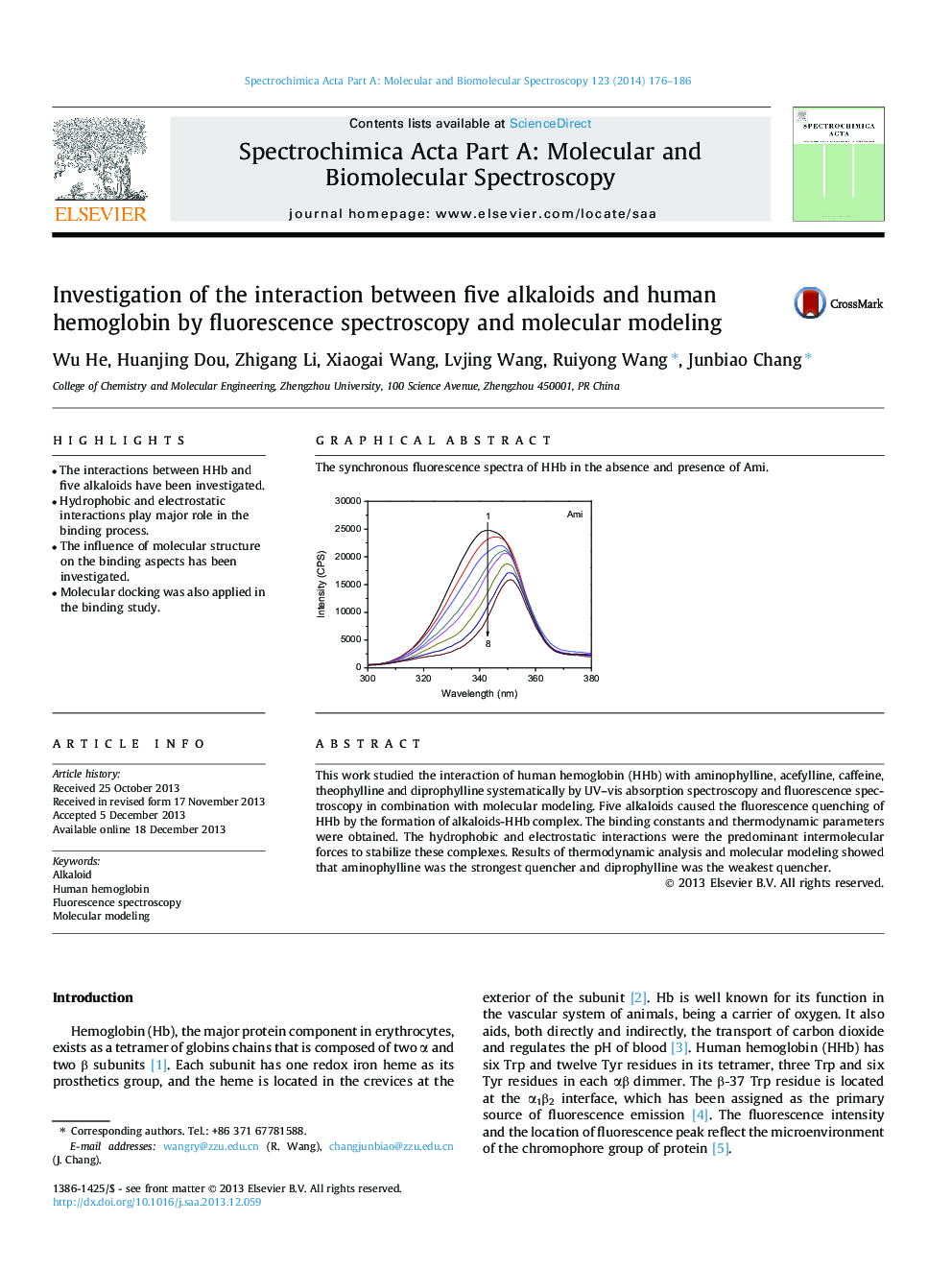
Graphical abstractThe synchronous fluorescence spectra of HHb in the absence and presence of Ami.Figure optionsDownload full-size imageDownload as PowerPoint slide