| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232838 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 5 Pages |
•The molecular vibration spectral bands of lanthanide ternary complexes were analyzed.•Wave-number of characteristic bands increases with increasing atomic number of Ln3+.•The vibration frequencies of C=O bonds were studied by the second derivative spectrum.
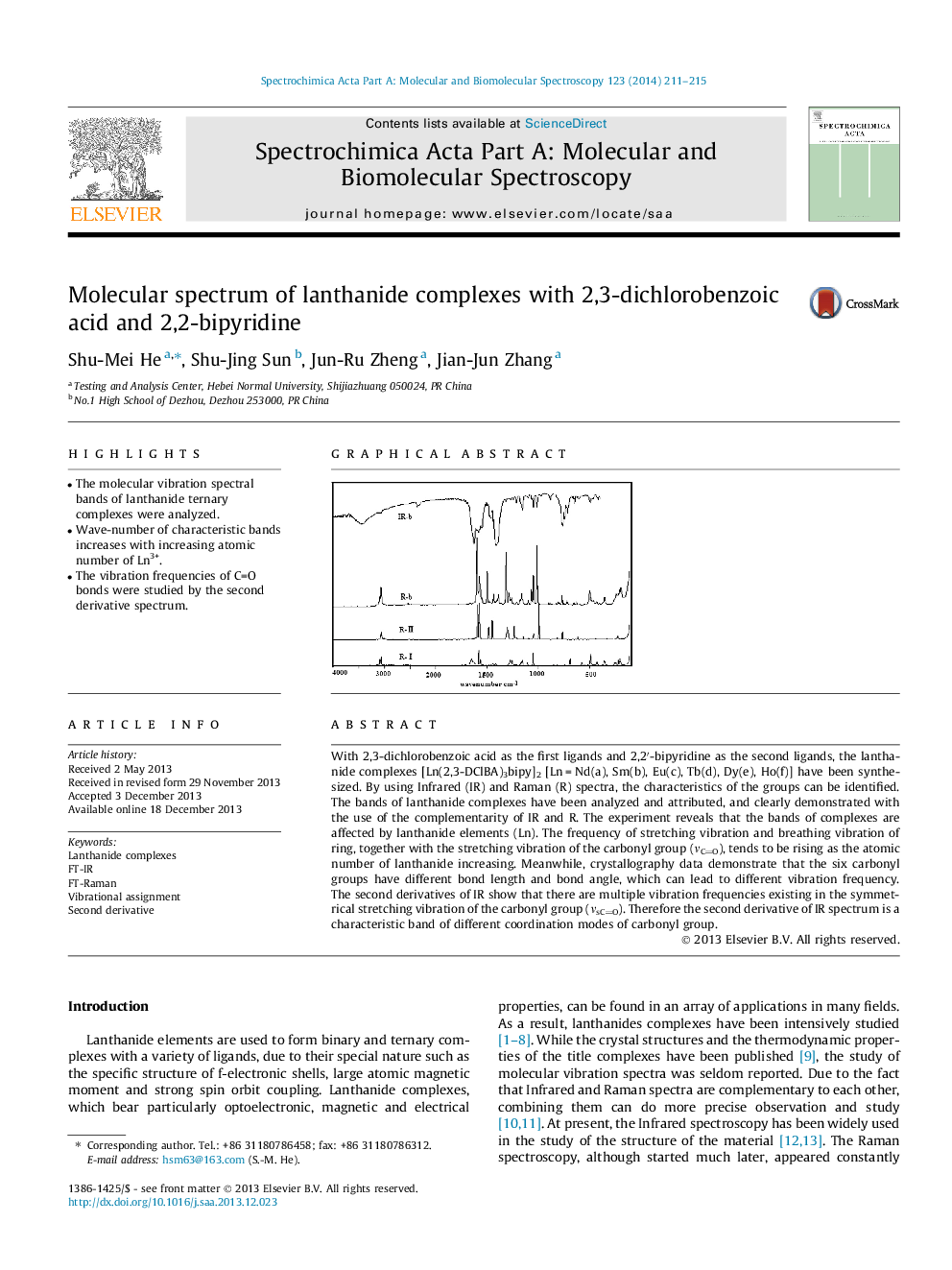
With 2,3-dichlorobenzoic acid as the first ligands and 2,2′-bipyridine as the second ligands, the lanthanide complexes [Ln(2,3-DClBA)3bipy]2 [Ln = Nd(a), Sm(b), Eu(c), Tb(d), Dy(e), Ho(f)] have been synthesized. By using Infrared (IR) and Raman (R) spectra, the characteristics of the groups can be identified. The bands of lanthanide complexes have been analyzed and attributed, and clearly demonstrated with the use of the complementarity of IR and R. The experiment reveals that the bands of complexes are affected by lanthanide elements (Ln). The frequency of stretching vibration and breathing vibration of ring, together with the stretching vibration of the carbonyl group (νCO), tends to be rising as the atomic number of lanthanide increasing. Meanwhile, crystallography data demonstrate that the six carbonyl groups have different bond length and bond angle, which can lead to different vibration frequency. The second derivatives of IR show that there are multiple vibration frequencies existing in the symmetrical stretching vibration of the carbonyl group (νsCO). Therefore the second derivative of IR spectrum is a characteristic band of different coordination modes of carbonyl group.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide