| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232844 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 10 Pages |
•New mono- and binuclear CuII complexes of a sulfamethazine Schiff-base were prepared.•Correlation between the experimental and theoretical work was performed.•Mononuclear complex is less toxic than free ligand, while the binuclear one is inactive.•ELUMO, ΔE, dipole moment and polarizability correlate well with experimental LC50.
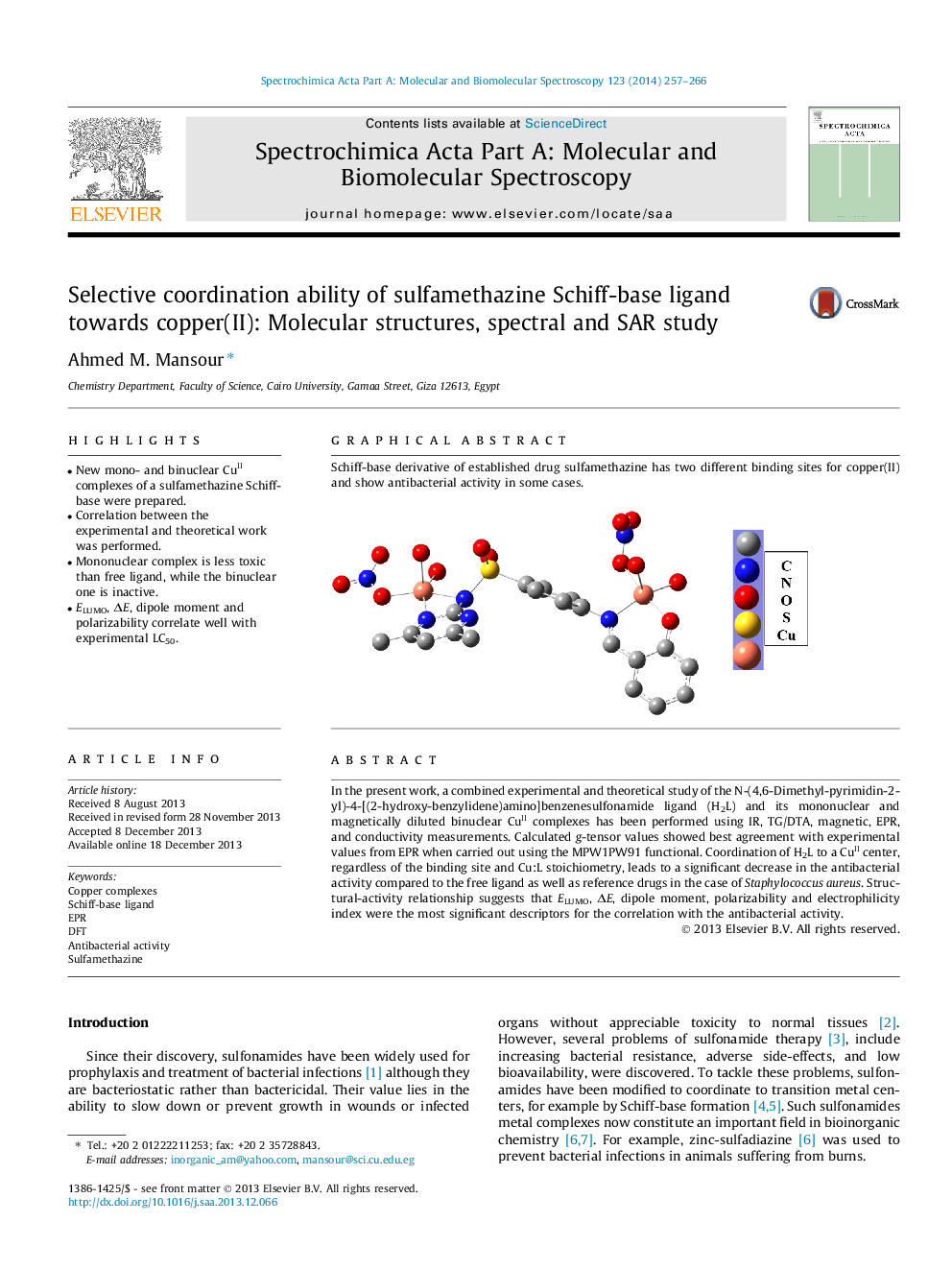
In the present work, a combined experimental and theoretical study of the N-(4,6-Dimethyl-pyrimidin-2-yl)-4-[(2-hydroxy-benzylidene)amino]benzenesulfonamide ligand (H2L) and its mononuclear and magnetically diluted binuclear CuII complexes has been performed using IR, TG/DTA, magnetic, EPR, and conductivity measurements. Calculated g-tensor values showed best agreement with experimental values from EPR when carried out using the MPW1PW91 functional. Coordination of H2L to a CuII center, regardless of the binding site and Cu:L stoichiometry, leads to a significant decrease in the antibacterial activity compared to the free ligand as well as reference drugs in the case of Staphylococcus aureus. Structural-activity relationship suggests that ELUMO, ΔE, dipole moment, polarizability and electrophilicity index were the most significant descriptors for the correlation with the antibacterial activity.
Graphical abstractSchiff-base derivative of established drug sulfamethazine has two different binding sites for copper(II) and show antibacterial activity in some cases.Figure optionsDownload full-size imageDownload as PowerPoint slide