| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232847 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 8 Pages |
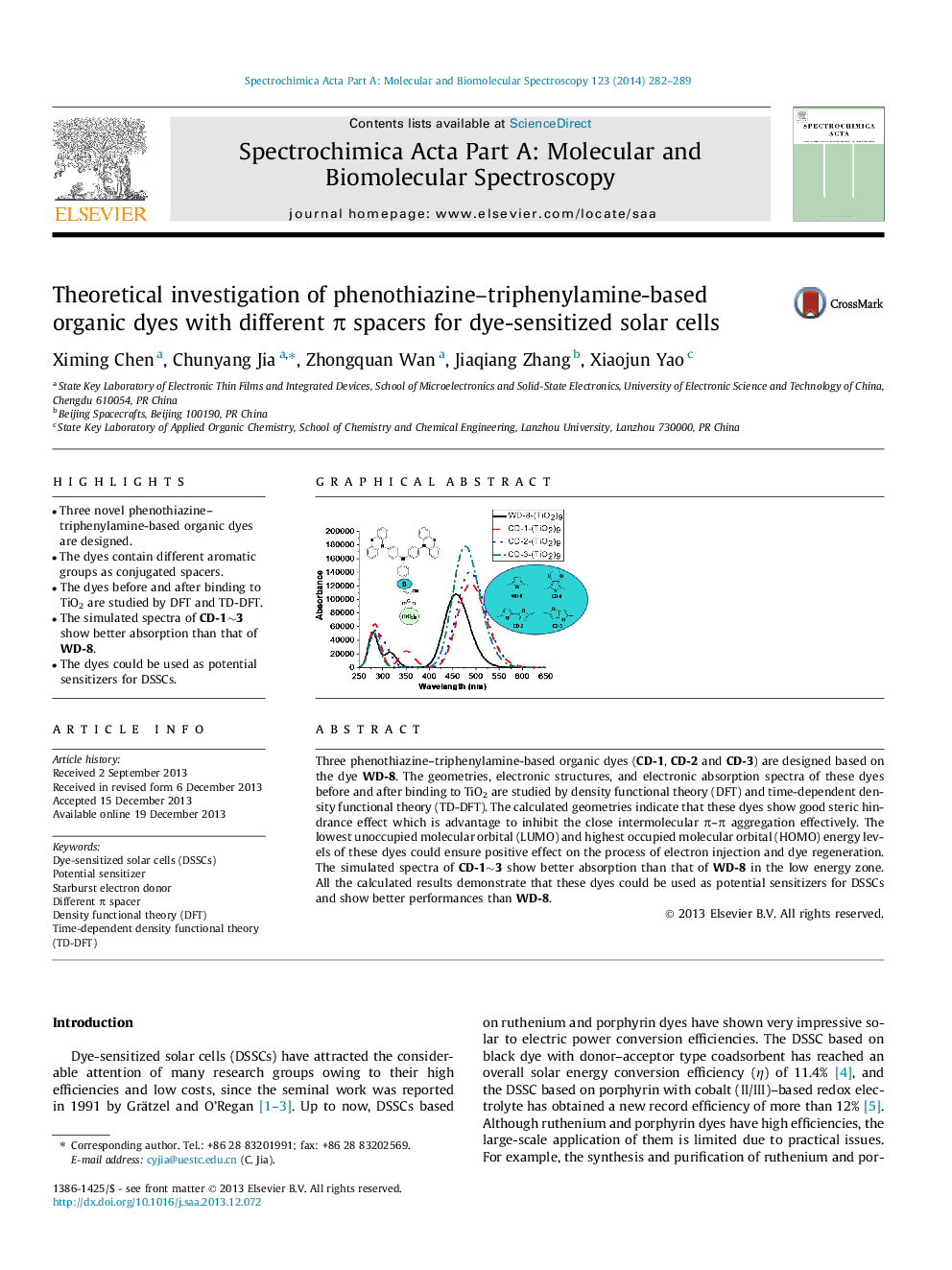
•Three novel phenothiazine–triphenylamine-based organic dyes are designed.•The dyes contain different aromatic groups as conjugated spacers.•The dyes before and after binding to TiO2 are studied by DFT and TD-DFT.•The simulated spectra of CD-1∼3 show better absorption than that of WD-8.•The dyes could be used as potential sensitizers for DSSCs.
Three phenothiazine–triphenylamine-based organic dyes (CD-1, CD-2 and CD-3) are designed based on the dye WD-8. The geometries, electronic structures, and electronic absorption spectra of these dyes before and after binding to TiO2 are studied by density functional theory (DFT) and time-dependent density functional theory (TD-DFT). The calculated geometries indicate that these dyes show good steric hindrance effect which is advantage to inhibit the close intermolecular π–π aggregation effectively. The lowest unoccupied molecular orbital (LUMO) and highest occupied molecular orbital (HOMO) energy levels of these dyes could ensure positive effect on the process of electron injection and dye regeneration. The simulated spectra of CD-1∼3 show better absorption than that of WD-8 in the low energy zone. All the calculated results demonstrate that these dyes could be used as potential sensitizers for DSSCs and show better performances than WD-8.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide