| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232850 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 6 Pages |
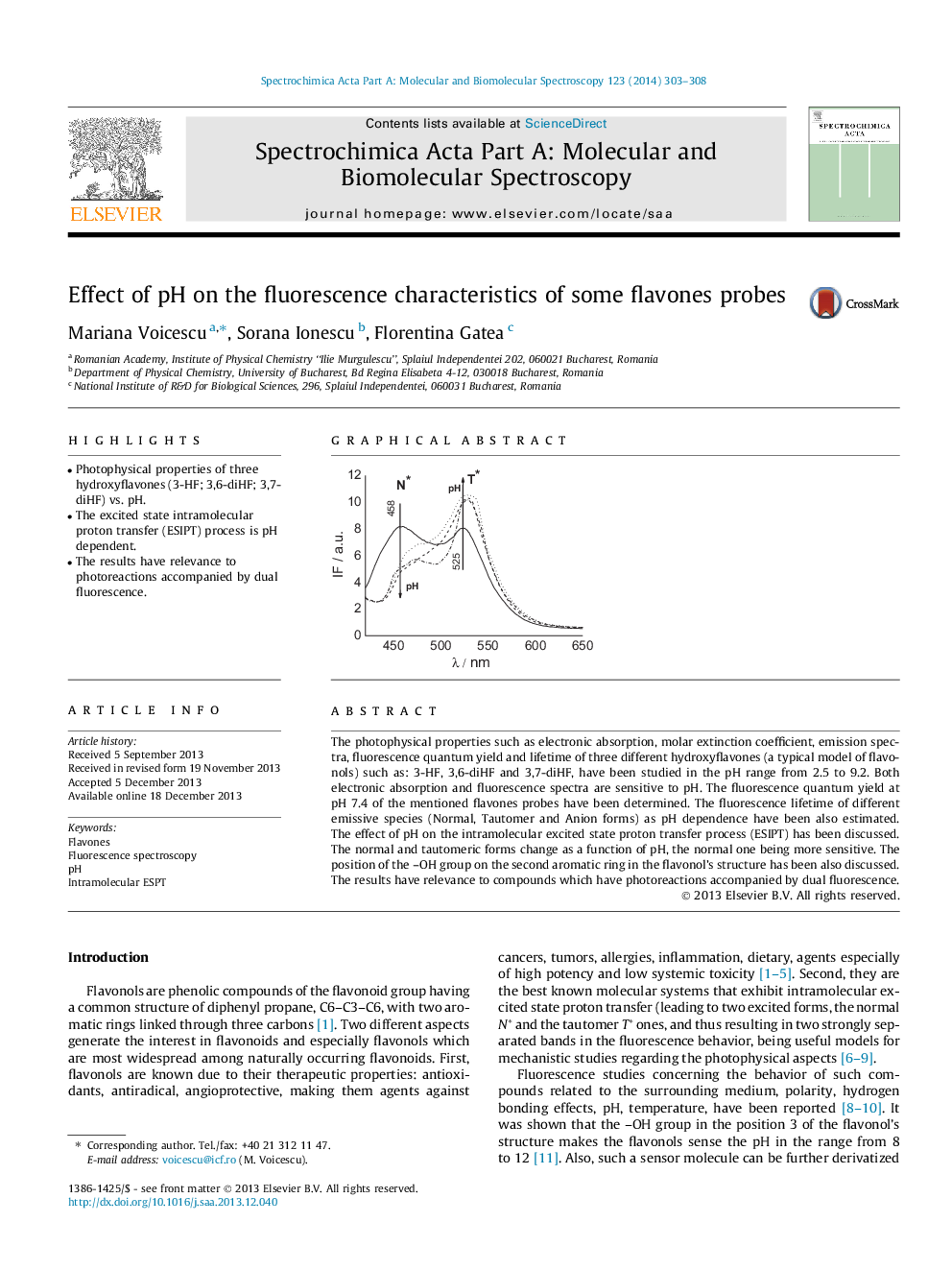
•Photophysical properties of three hydroxyflavones (3-HF; 3,6-diHF; 3,7-diHF) vs. pH.•The excited state intramolecular proton transfer (ESIPT) process is pH dependent.•The results have relevance to photoreactions accompanied by dual fluorescence.
The photophysical properties such as electronic absorption, molar extinction coefficient, emission spectra, fluorescence quantum yield and lifetime of three different hydroxyflavones (a typical model of flavonols) such as: 3-HF, 3,6-diHF and 3,7-diHF, have been studied in the pH range from 2.5 to 9.2. Both electronic absorption and fluorescence spectra are sensitive to pH. The fluorescence quantum yield at pH 7.4 of the mentioned flavones probes have been determined. The fluorescence lifetime of different emissive species (Normal, Tautomer and Anion forms) as pH dependence have been also estimated. The effect of pH on the intramolecular excited state proton transfer process (ESIPT) has been discussed. The normal and tautomeric forms change as a function of pH, the normal one being more sensitive. The position of the –OH group on the second aromatic ring in the flavonol’s structure has been also discussed. The results have relevance to compounds which have photoreactions accompanied by dual fluorescence.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide