| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232855 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 11 Pages |
•Monomeric and dimeric conformations of 3,5-dimethyl-4-methoxybenzoic acid were investigated.•The compound was characterized by FT-IR and FT-Raman, 1H, 13C NMR and UV spectroscopy.•The vibrational frequencies, chemical shifts and electronic absorption wavelengths were calculated by DFT method.•The NLO properties and NBO analyses were addressed theoretically.
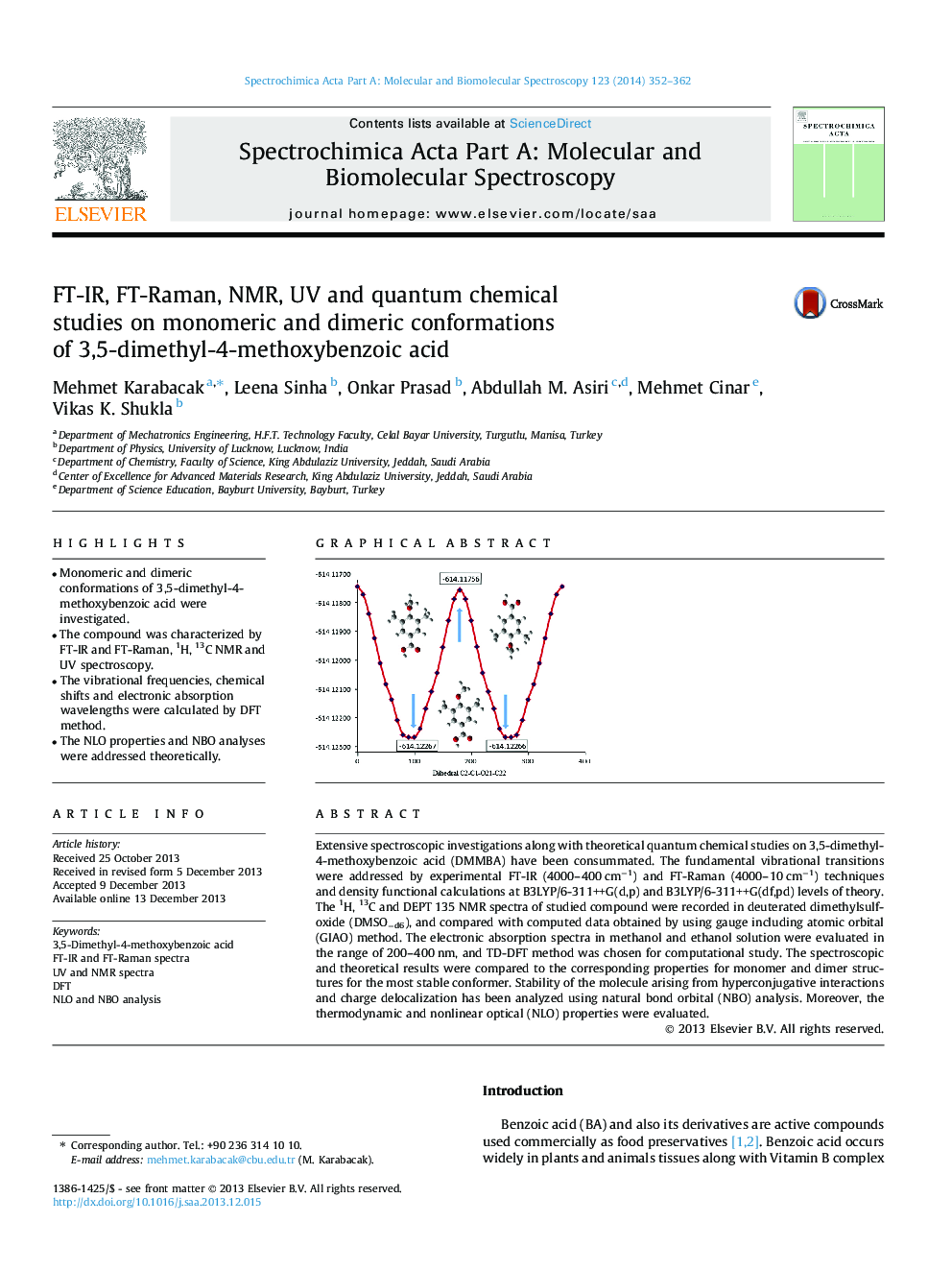
Extensive spectroscopic investigations along with theoretical quantum chemical studies on 3,5-dimethyl-4-methoxybenzoic acid (DMMBA) have been consummated. The fundamental vibrational transitions were addressed by experimental FT-IR (4000–400 cm−1) and FT-Raman (4000–10 cm−1) techniques and density functional calculations at B3LYP/6-311++G(d,p) and B3LYP/6-311++G(df,pd) levels of theory. The 1H, 13C and DEPT 135 NMR spectra of studied compound were recorded in deuterated dimethylsulfoxide (DMSO−d6), and compared with computed data obtained by using gauge including atomic orbital (GIAO) method. The electronic absorption spectra in methanol and ethanol solution were evaluated in the range of 200–400 nm, and TD-DFT method was chosen for computational study. The spectroscopic and theoretical results were compared to the corresponding properties for monomer and dimer structures for the most stable conformer. Stability of the molecule arising from hyperconjugative interactions and charge delocalization has been analyzed using natural bond orbital (NBO) analysis. Moreover, the thermodynamic and nonlinear optical (NLO) properties were evaluated.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide