| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232859 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 7 Pages |
•Adsorption of organic solvents vapours on heated Cu-montmorillonites was examined.•The adsorbed amount was obtained from 2νCH bands areas in the NIR region.•DMSO fully solvated all samples prepared at 60–300 °C.•Acetonitrile only partially solvated samples prepared at 300 °C.•Fixation of Cu2+ in montmorillonites upon heating is partially reversible.
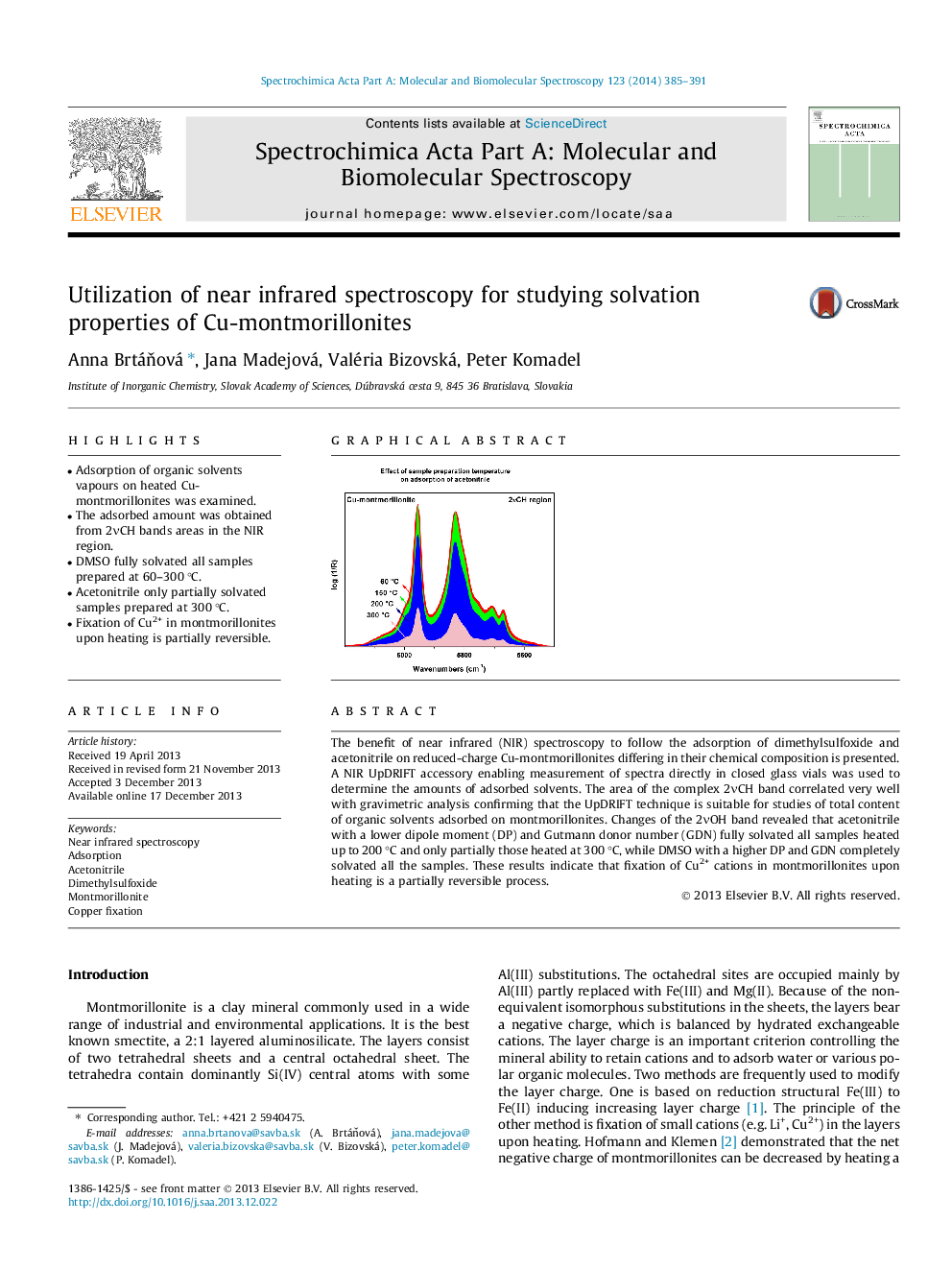
The benefit of near infrared (NIR) spectroscopy to follow the adsorption of dimethylsulfoxide and acetonitrile on reduced-charge Cu-montmorillonites differing in their chemical composition is presented. A NIR UpDRIFT accessory enabling measurement of spectra directly in closed glass vials was used to determine the amounts of adsorbed solvents. The area of the complex 2νCH band correlated very well with gravimetric analysis confirming that the UpDRIFT technique is suitable for studies of total content of organic solvents adsorbed on montmorillonites. Changes of the 2νOH band revealed that acetonitrile with a lower dipole moment (DP) and Gutmann donor number (GDN) fully solvated all samples heated up to 200 °C and only partially those heated at 300 °C, while DMSO with a higher DP and GDN completely solvated all the samples. These results indicate that fixation of Cu2+ cations in montmorillonites upon heating is a partially reversible process.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide