| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232864 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 9 Pages |
•Anion induced tautomerism of isatinphenylsemicarbazone sensors was investigated.•Acid–base properties of anions influence the equilibrium ratio of the individual tautomeric forms.•Sensor E and Z isomers differs in sensitivity, selectivity and sensing mechanism.•Sensors provide an excellent signal to noise ratio and also wide detection range.•Detection of strongly basic anions in both organic and semi-aqueous media is possible.
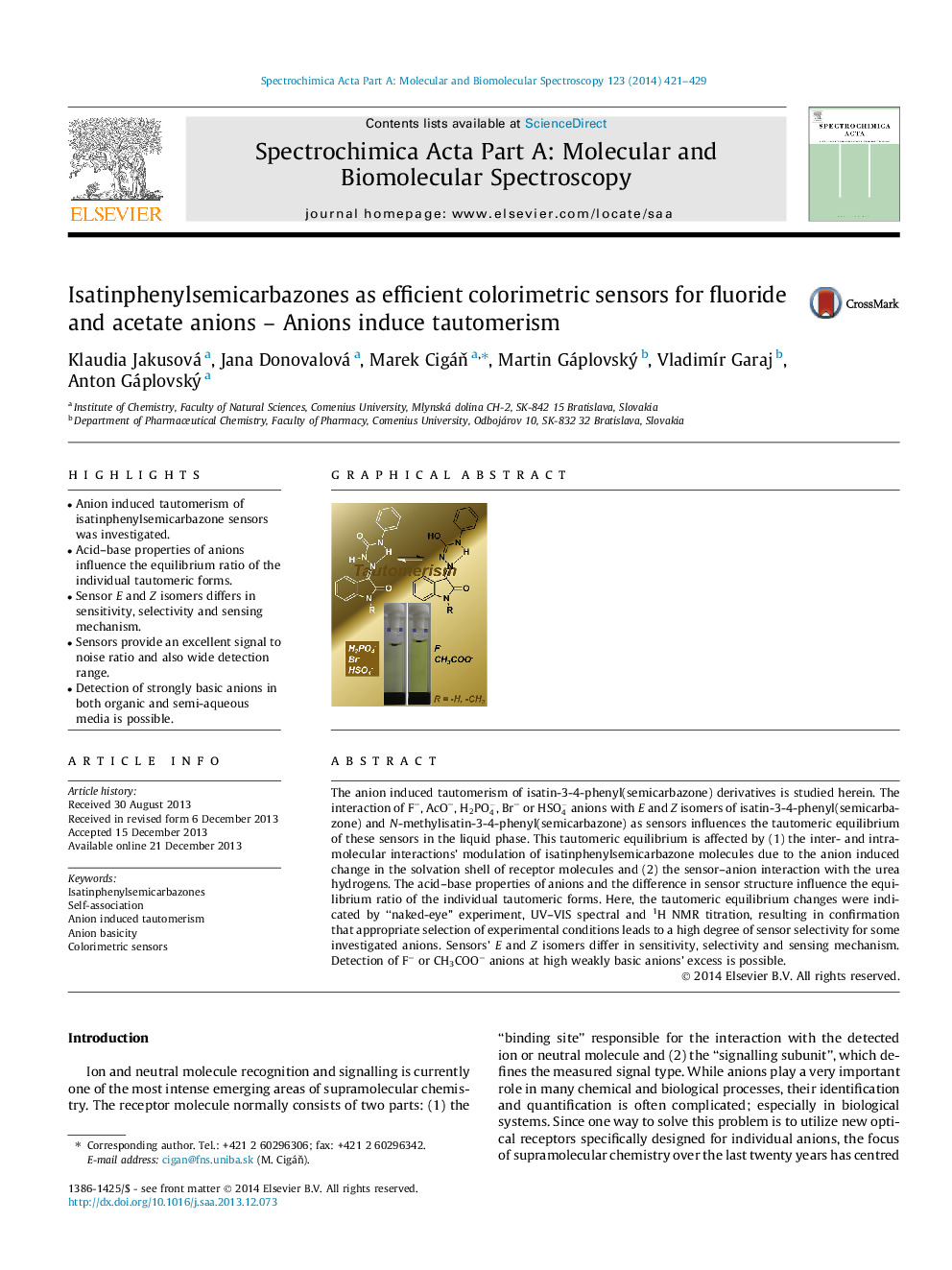
The anion induced tautomerism of isatin-3-4-phenyl(semicarbazone) derivatives is studied herein. The interaction of F−, AcO−, H2PO4-, Br− or HSO4- anions with E and Z isomers of isatin-3-4-phenyl(semicarbazone) and N-methylisatin-3-4-phenyl(semicarbazone) as sensors influences the tautomeric equilibrium of these sensors in the liquid phase. This tautomeric equilibrium is affected by (1) the inter- and intra-molecular interactions’ modulation of isatinphenylsemicarbazone molecules due to the anion induced change in the solvation shell of receptor molecules and (2) the sensor–anion interaction with the urea hydrogens. The acid–base properties of anions and the difference in sensor structure influence the equilibrium ratio of the individual tautomeric forms. Here, the tautomeric equilibrium changes were indicated by “naked-eye” experiment, UV–VIS spectral and 1H NMR titration, resulting in confirmation that appropriate selection of experimental conditions leads to a high degree of sensor selectivity for some investigated anions. Sensors’ E and Z isomers differ in sensitivity, selectivity and sensing mechanism. Detection of F− or CH3COO− anions at high weakly basic anions’ excess is possible.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide