| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232867 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 8 Pages |
•The structure of indomethacin is optimized at the DFT and MP2 levels.•The vibrational wavenumbers of the drug were calculated at the DFT level.•Vibrational assignments were provided by combining experimental and theoretical data.•The vibrational analysis is consistent with the presence of a single conformer in the spectra of solid indomethacin.
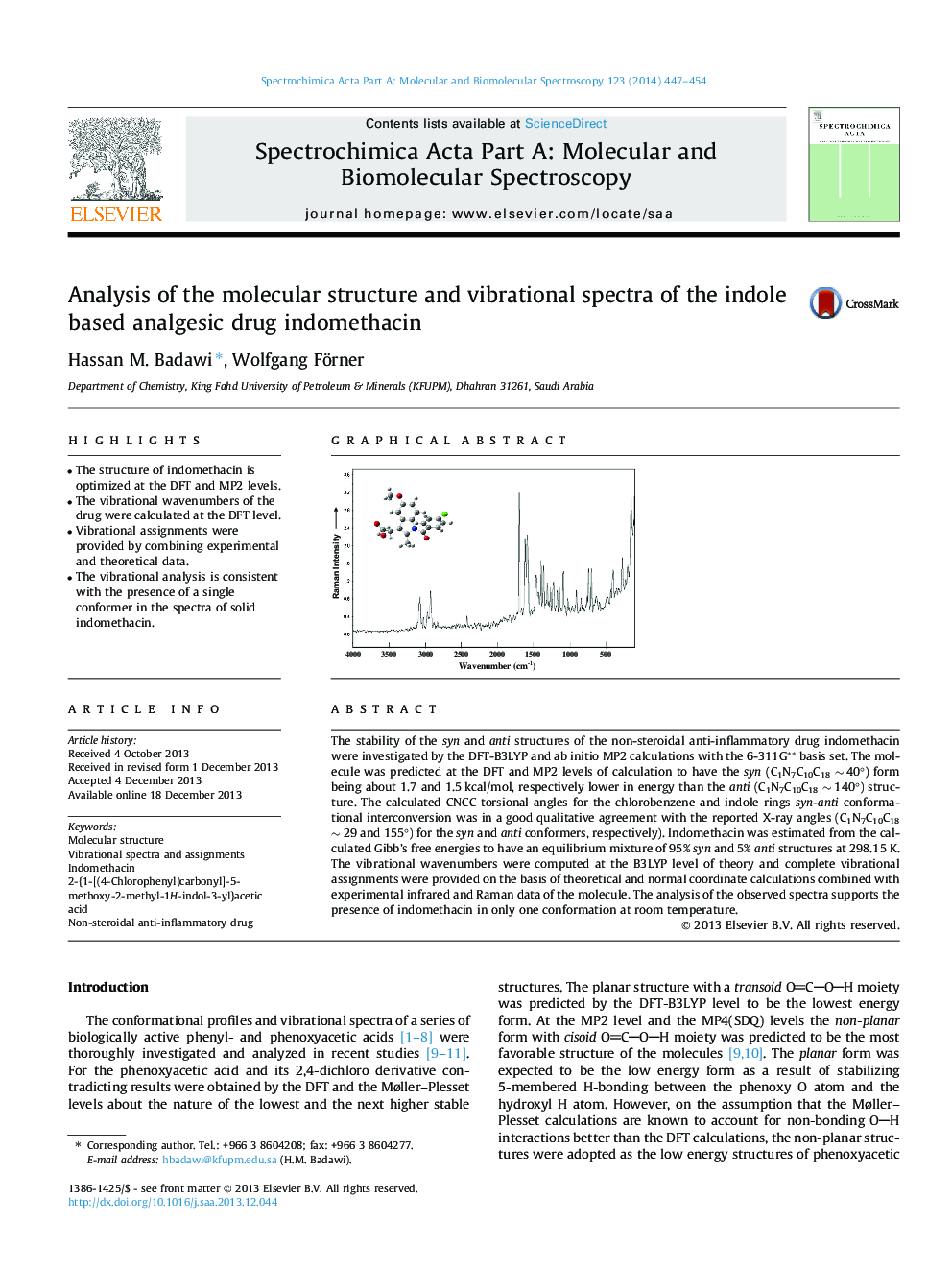
The stability of the syn and anti structures of the non-steroidal anti-inflammatory drug indomethacin were investigated by the DFT-B3LYP and ab initio MP2 calculations with the 6-311G** basis set. The molecule was predicted at the DFT and MP2 levels of calculation to have the syn (C1N7C10C18 ∼ 40°) form being about 1.7 and 1.5 kcal/mol, respectively lower in energy than the anti (C1N7C10C18 ∼ 140°) structure. The calculated CNCC torsional angles for the chlorobenzene and indole rings syn-anti conformational interconversion was in a good qualitative agreement with the reported X-ray angles (C1N7C10C18 ∼ 29 and 155°) for the syn and anti conformers, respectively). Indomethacin was estimated from the calculated Gibb’s free energies to have an equilibrium mixture of 95% syn and 5% anti structures at 298.15 K. The vibrational wavenumbers were computed at the B3LYP level of theory and complete vibrational assignments were provided on the basis of theoretical and normal coordinate calculations combined with experimental infrared and Raman data of the molecule. The analysis of the observed spectra supports the presence of indomethacin in only one conformation at room temperature.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide