| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1232875 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 13 Pages |
•NBO analysis provides information about delocalization charge of mesogen.•The observed and theoretical FT-IR spectra corroborated the H- bond in the mesogen.•Analysis of energy gap revealed the reactivity of all the reactants.•Solvent effect investigations provide dipole moment and stability of mesogen.•Nematic phase stability of mesogen is evidenced by local charge distribution.
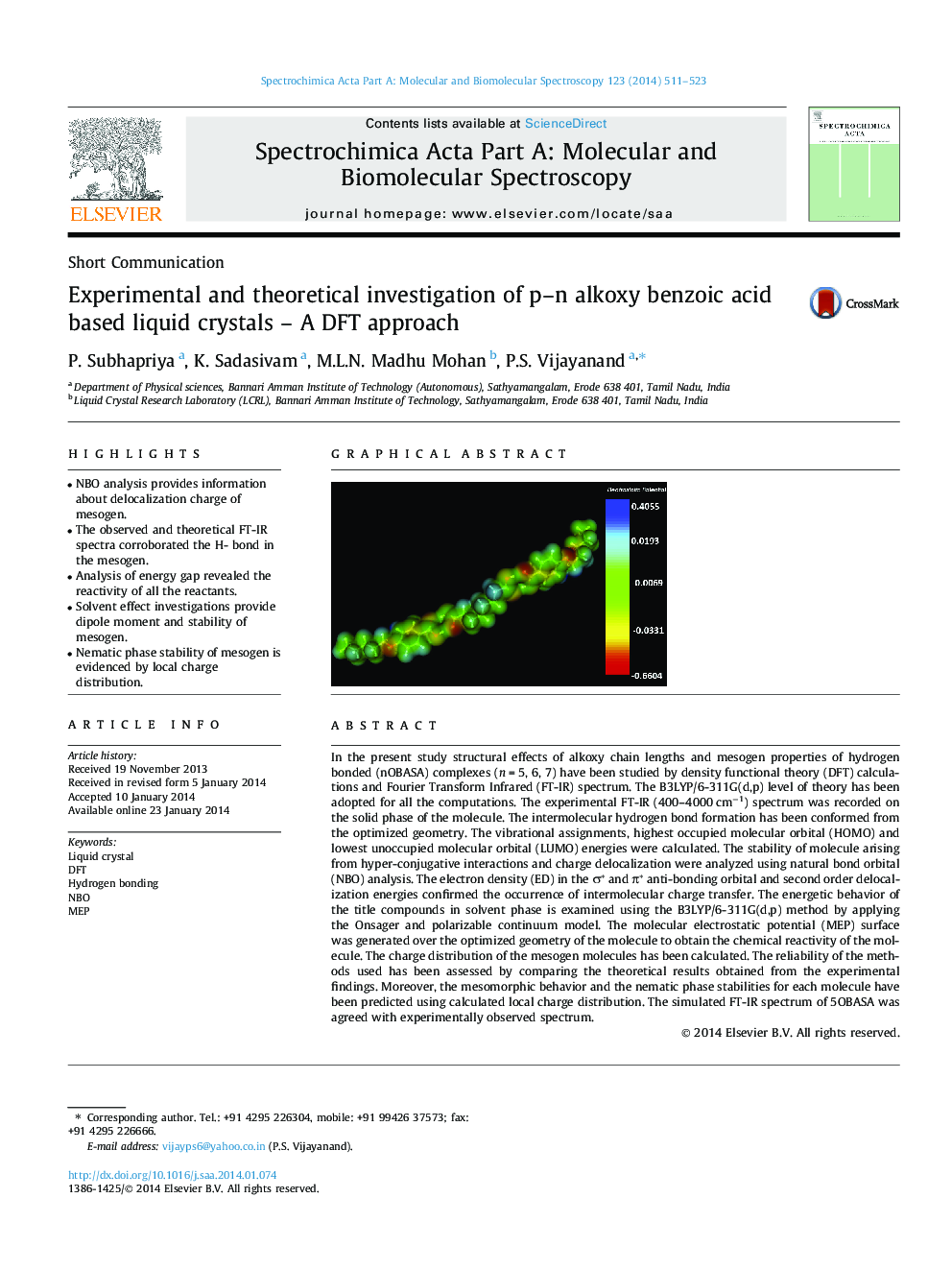
In the present study structural effects of alkoxy chain lengths and mesogen properties of hydrogen bonded (nOBASA) complexes (n = 5, 6, 7) have been studied by density functional theory (DFT) calculations and Fourier Transform Infrared (FT-IR) spectrum. The B3LYP/6-311G(d,p) level of theory has been adopted for all the computations. The experimental FT-IR (400–4000 cm−1) spectrum was recorded on the solid phase of the molecule. The intermolecular hydrogen bond formation has been conformed from the optimized geometry. The vibrational assignments, highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energies were calculated. The stability of molecule arising from hyper-conjugative interactions and charge delocalization were analyzed using natural bond orbital (NBO) analysis. The electron density (ED) in the σ* and π* anti-bonding orbital and second order delocalization energies confirmed the occurrence of intermolecular charge transfer. The energetic behavior of the title compounds in solvent phase is examined using the B3LYP/6-311G(d,p) method by applying the Onsager and polarizable continuum model. The molecular electrostatic potential (MEP) surface was generated over the optimized geometry of the molecule to obtain the chemical reactivity of the molecule. The charge distribution of the mesogen molecules has been calculated. The reliability of the methods used has been assessed by comparing the theoretical results obtained from the experimental findings. Moreover, the mesomorphic behavior and the nematic phase stabilities for each molecule have been predicted using calculated local charge distribution. The simulated FT-IR spectrum of 5OBASA was agreed with experimentally observed spectrum.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide