| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233082 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 8 Pages |
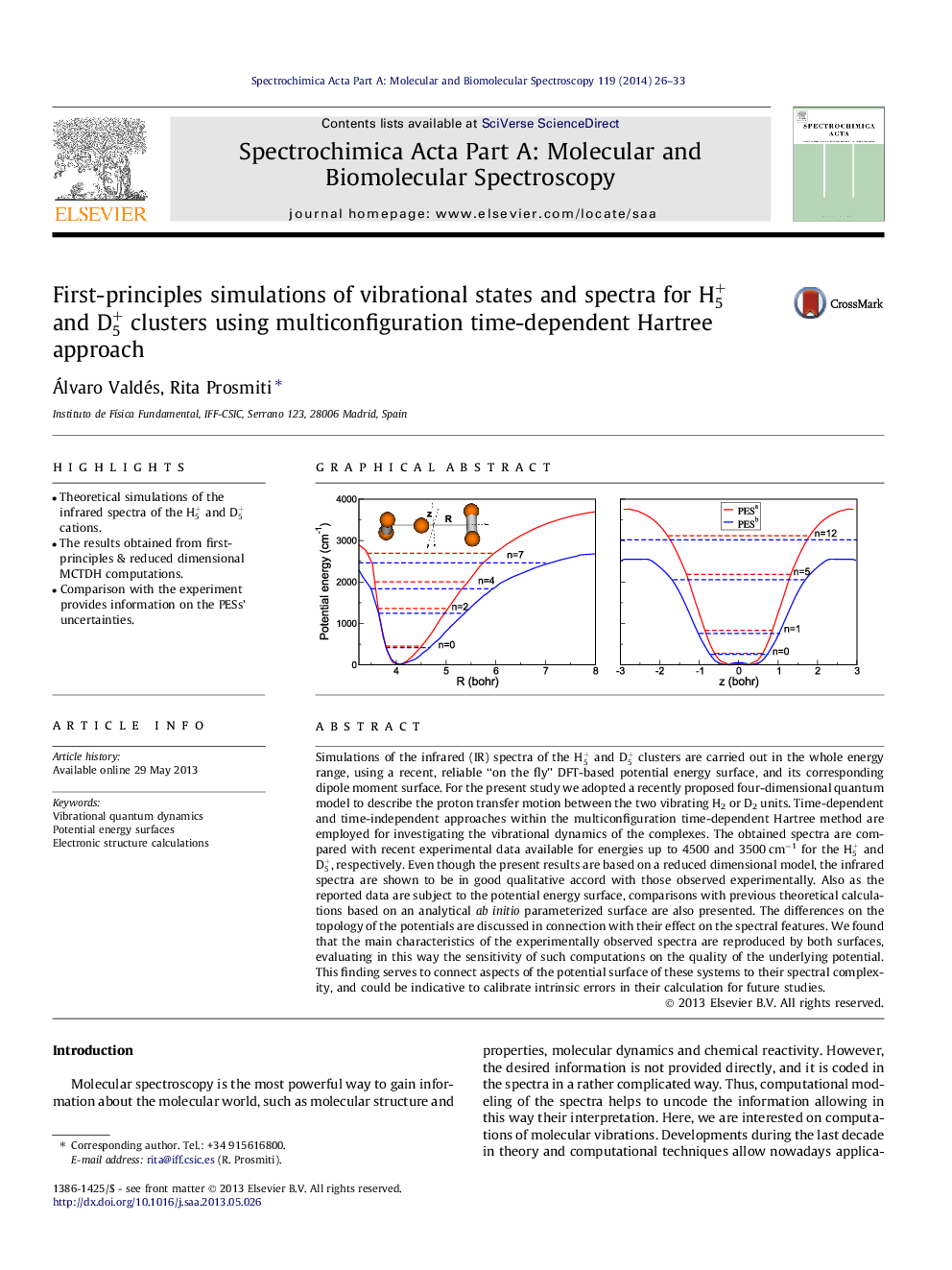
•Theoretical simulations of the infrared spectra of the H5+ and D5+ cations.•The results obtained from first-principles & reduced dimensional MCTDH computations.•Comparison with the experiment provides information on the PESs’ uncertainties.
Simulations of the infrared (IR) spectra of the H5+ and D5+ clusters are carried out in the whole energy range, using a recent, reliable “on the fly” DFT-based potential energy surface, and its corresponding dipole moment surface. For the present study we adopted a recently proposed four-dimensional quantum model to describe the proton transfer motion between the two vibrating H2 or D2 units. Time-dependent and time-independent approaches within the multiconfiguration time-dependent Hartree method are employed for investigating the vibrational dynamics of the complexes. The obtained spectra are compared with recent experimental data available for energies up to 4500 and 3500 cm−1 for the H5+ and D5+, respectively. Even though the present results are based on a reduced dimensional model, the infrared spectra are shown to be in good qualitative accord with those observed experimentally. Also as the reported data are subject to the potential energy surface, comparisons with previous theoretical calculations based on an analytical ab initio parameterized surface are also presented. The differences on the topology of the potentials are discussed in connection with their effect on the spectral features. We found that the main characteristics of the experimentally observed spectra are reproduced by both surfaces, evaluating in this way the sensitivity of such computations on the quality of the underlying potential. This finding serves to connect aspects of the potential surface of these systems to their spectral complexity, and could be indicative to calibrate intrinsic errors in their calculation for future studies.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide