| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233156 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 7 Pages |
•Novel indolizine derivatives were prepared by tandem reaction and fully characterized.•UV–vis absorption and fluorescence spectroscopy of all compounds were measured.•Influence of solvent and substituent on UV–vis absorption and fluorescence spectroscopy was examined.
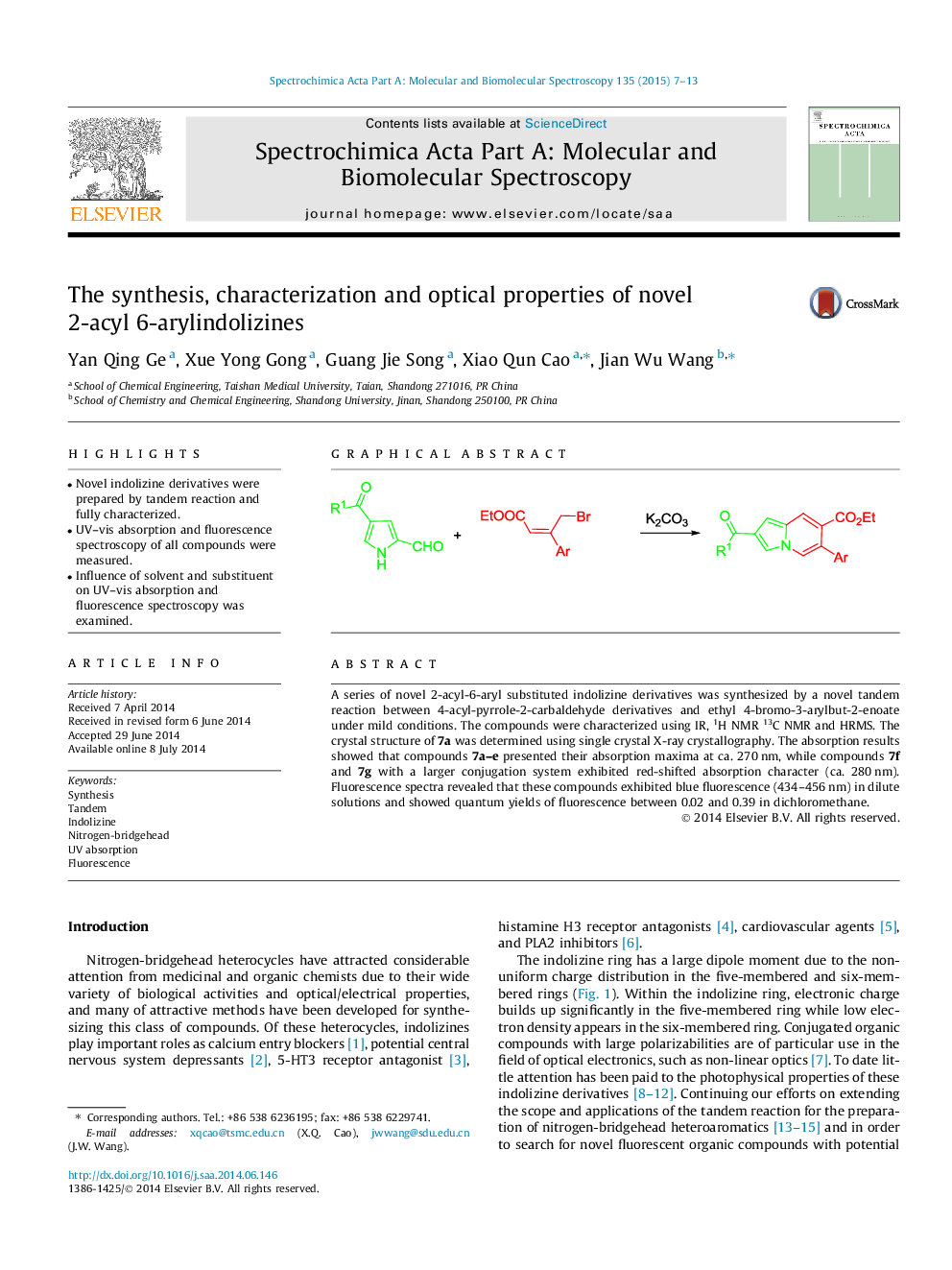
A series of novel 2-acyl-6-aryl substituted indolizine derivatives was synthesized by a novel tandem reaction between 4-acyl-pyrrole-2-carbaldehyde derivatives and ethyl 4-bromo-3-arylbut-2-enoate under mild conditions. The compounds were characterized using IR, 1H NMR 13C NMR and HRMS. The crystal structure of 7a was determined using single crystal X-ray crystallography. The absorption results showed that compounds 7a–e presented their absorption maxima at ca. 270 nm, while compounds 7f and 7g with a larger conjugation system exhibited red-shifted absorption character (ca. 280 nm). Fluorescence spectra revealed that these compounds exhibited blue fluorescence (434–456 nm) in dilute solutions and showed quantum yields of fluorescence between 0.02 and 0.39 in dichloromethane.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide