| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233164 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 6 Pages |
•Two truxene-cored asymmetric multibranched π-conjugated molecules were synthesized.•The two compounds exhibit almost same absorption maxima, but different emission.•The theoretical results reveal the HOMO → LUMO transition is not their main transition.
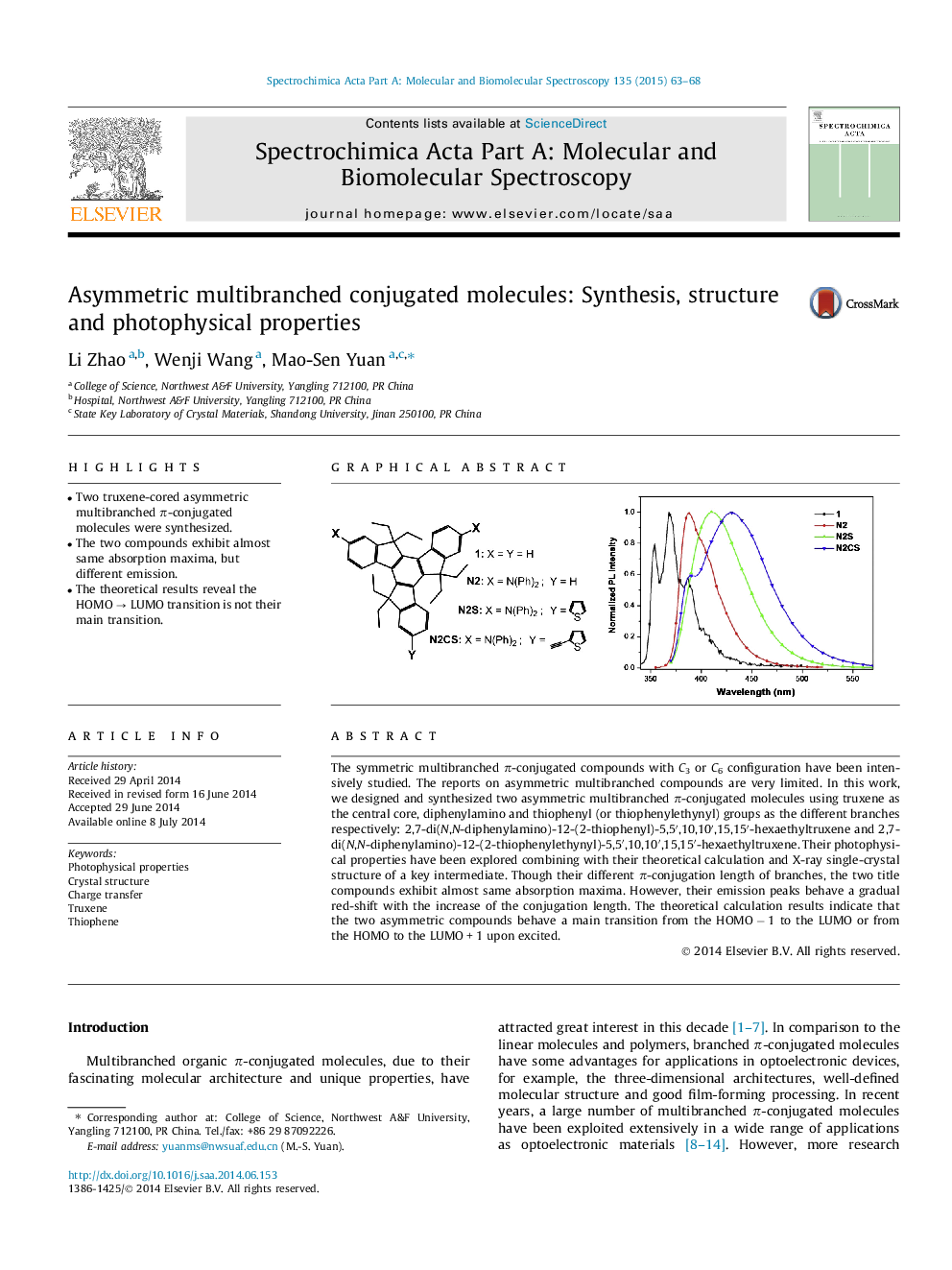
The symmetric multibranched π-conjugated compounds with C3 or C6 configuration have been intensively studied. The reports on asymmetric multibranched compounds are very limited. In this work, we designed and synthesized two asymmetric multibranched π-conjugated molecules using truxene as the central core, diphenylamino and thiophenyl (or thiophenylethynyl) groups as the different branches respectively: 2,7-di(N,N-diphenylamino)-12-(2-thiophenyl)-5,5′,10,10′,15,15′-hexaethyltruxene and 2,7-di(N,N-diphenylamino)-12-(2-thiophenylethynyl)-5,5′,10,10′,15,15′-hexaethyltruxene. Their photophysical properties have been explored combining with their theoretical calculation and X-ray single-crystal structure of a key intermediate. Though their different π-conjugation length of branches, the two title compounds exhibit almost same absorption maxima. However, their emission peaks behave a gradual red-shift with the increase of the conjugation length. The theoretical calculation results indicate that the two asymmetric compounds behave a main transition from the HOMO − 1 to the LUMO or from the HOMO to the LUMO + 1 upon excited.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide