| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233175 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 9 Pages |
•Green synthesis of silver nanoparticles using Ficus carica leaf extracts.•Effects of irradiation on reduction rate and nanoparticle size are investigated.•Irradiance, not wavelength, is influential on reduction rate and cluster size.•Particle shape and size distribution do not change with irradiance and wavelength.•Role of alkanes, alkenes, alkynes, amines, alcohols and phenols in Ag+ ion reduction.
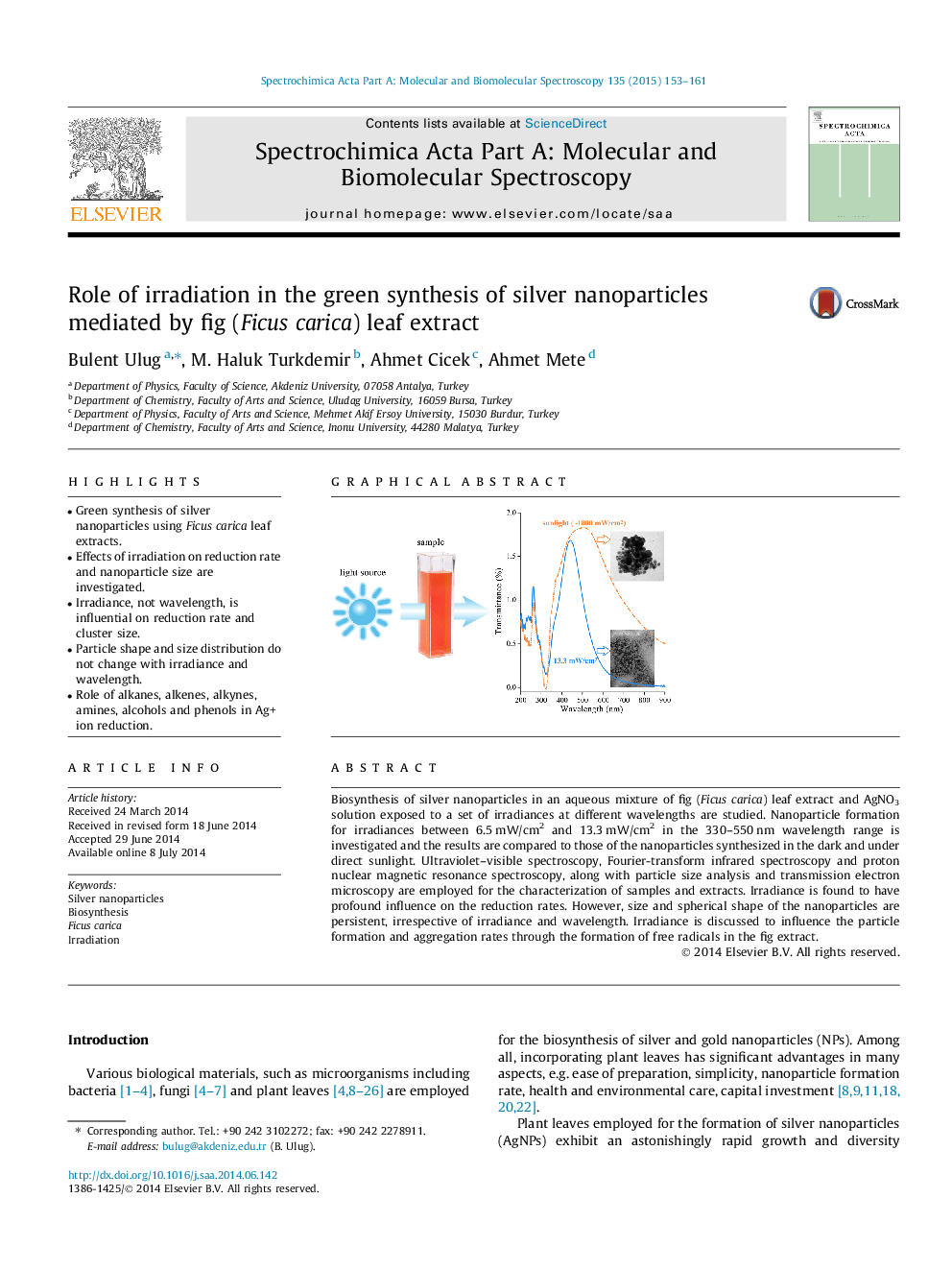
Biosynthesis of silver nanoparticles in an aqueous mixture of fig (Ficus carica) leaf extract and AgNO3 solution exposed to a set of irradiances at different wavelengths are studied. Nanoparticle formation for irradiances between 6.5 mW/cm2 and 13.3 mW/cm2 in the 330–550 nm wavelength range is investigated and the results are compared to those of the nanoparticles synthesized in the dark and under direct sunlight. Ultraviolet–visible spectroscopy, Fourier-transform infrared spectroscopy and proton nuclear magnetic resonance spectroscopy, along with particle size analysis and transmission electron microscopy are employed for the characterization of samples and extracts. Irradiance is found to have profound influence on the reduction rates. However, size and spherical shape of the nanoparticles are persistent, irrespective of irradiance and wavelength. Irradiance is discussed to influence the particle formation and aggregation rates through the formation of free radicals in the fig extract.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide