| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233186 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 11 Pages |
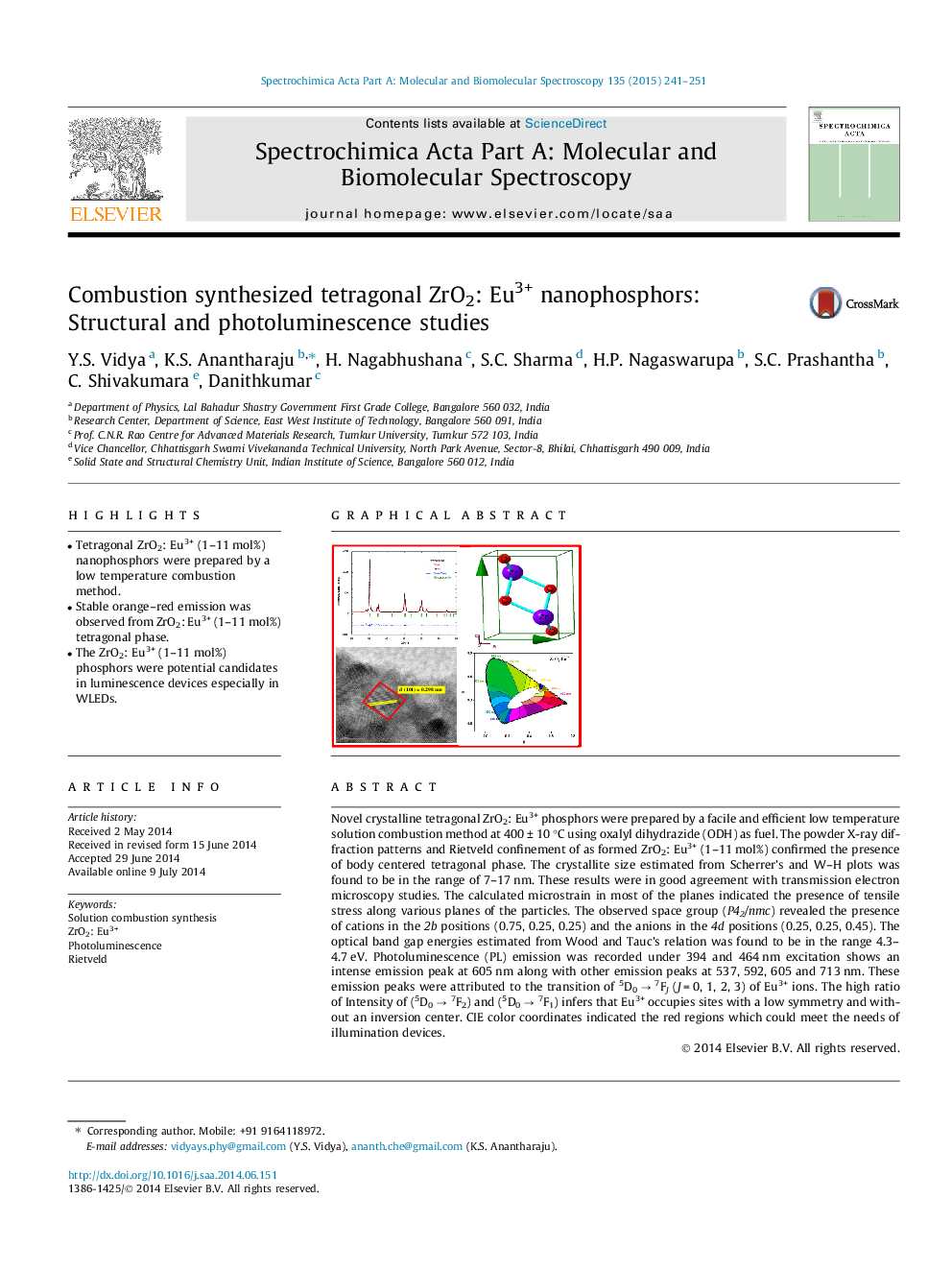
•Tetragonal ZrO2: Eu3+ (1–11 mol%) nanophosphors were prepared by a low temperature combustion method.•Stable orange–red emission was observed from ZrO2: Eu3+ (1–11 mol%) tetragonal phase.•The ZrO2: Eu3+ (1–11 mol%) phosphors were potential candidates in luminescence devices especially in WLEDs.
Novel crystalline tetragonal ZrO2: Eu3+ phosphors were prepared by a facile and efficient low temperature solution combustion method at 400 ± 10 °C using oxalyl dihydrazide (ODH) as fuel. The powder X-ray diffraction patterns and Rietveld confinement of as formed ZrO2: Eu3+ (1–11 mol%) confirmed the presence of body centered tetragonal phase. The crystallite size estimated from Scherrer’s and W–H plots was found to be in the range of 7–17 nm. These results were in good agreement with transmission electron microscopy studies. The calculated microstrain in most of the planes indicated the presence of tensile stress along various planes of the particles. The observed space group (P42/nmc) revealed the presence of cations in the 2b positions (0.75, 0.25, 0.25) and the anions in the 4d positions (0.25, 0.25, 0.45). The optical band gap energies estimated from Wood and Tauc’s relation was found to be in the range 4.3–4.7 eV. Photoluminescence (PL) emission was recorded under 394 and 464 nm excitation shows an intense emission peak at 605 nm along with other emission peaks at 537, 592, 605 and 713 nm. These emission peaks were attributed to the transition of 5D0 → 7FJ (J = 0, 1, 2, 3) of Eu3+ ions. The high ratio of Intensity of (5D0 → 7F2) and (5D0 → 7F1) infers that Eu3+ occupies sites with a low symmetry and without an inversion center. CIE color coordinates indicated the red regions which could meet the needs of illumination devices.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide