| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233190 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 13 Pages |
•Monomeric and anion conformations and dimeric structure of 3,4-PDCA were investigated.•Spectroscopic features of 3,4-PDCA were examined by NMR, UV, infrared and Raman techniques.•The vibrational frequencies, chemical shifts and electronic absorption wavelengths were calculated by DFT.
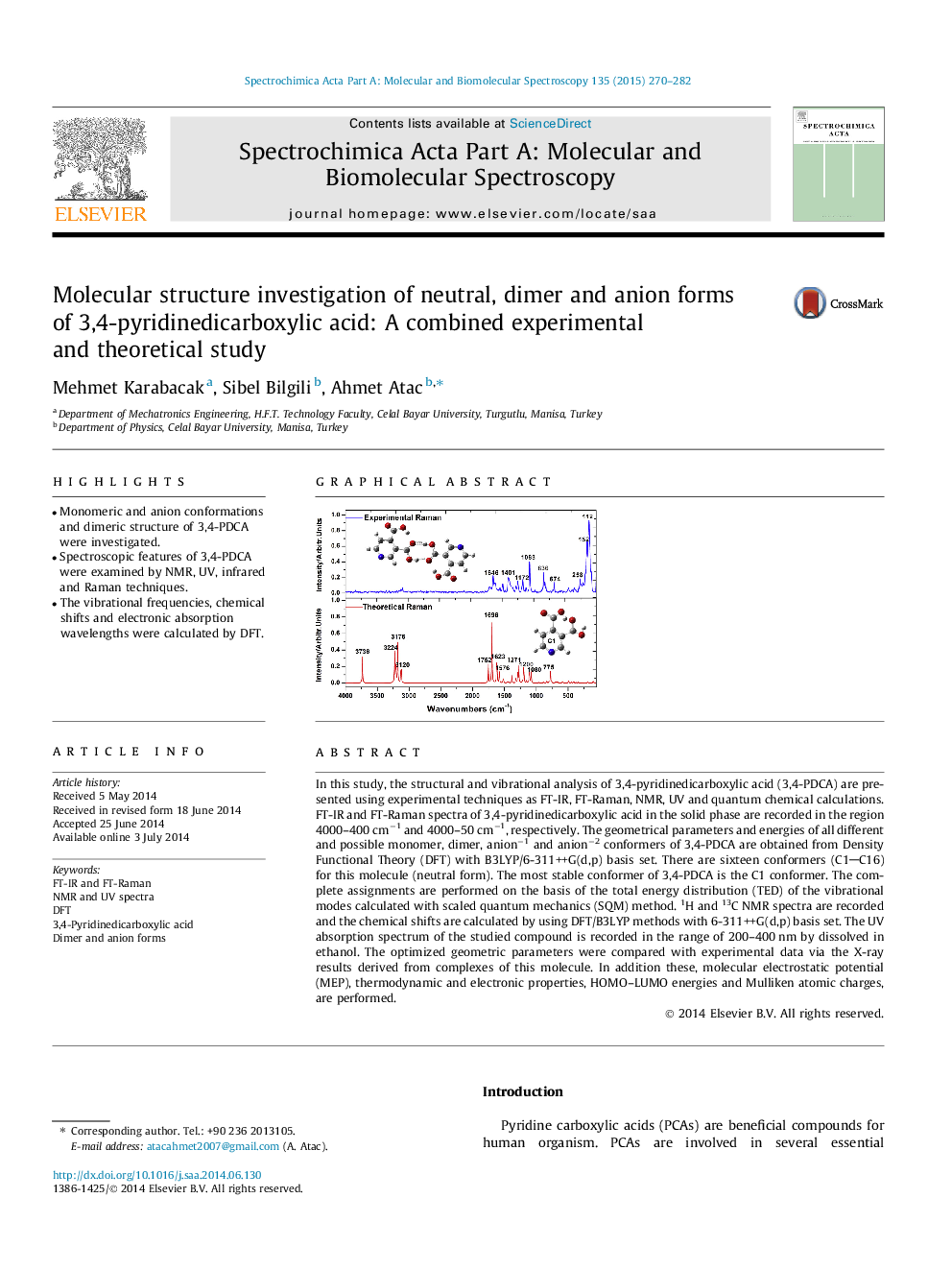
In this study, the structural and vibrational analysis of 3,4-pyridinedicarboxylic acid (3,4-PDCA) are presented using experimental techniques as FT-IR, FT-Raman, NMR, UV and quantum chemical calculations. FT-IR and FT-Raman spectra of 3,4-pyridinedicarboxylic acid in the solid phase are recorded in the region 4000–400 cm−1 and 4000–50 cm−1, respectively. The geometrical parameters and energies of all different and possible monomer, dimer, anion−1 and anion−2 conformers of 3,4-PDCA are obtained from Density Functional Theory (DFT) with B3LYP/6-311++G(d,p) basis set. There are sixteen conformers (C1C16) for this molecule (neutral form). The most stable conformer of 3,4-PDCA is the C1 conformer. The complete assignments are performed on the basis of the total energy distribution (TED) of the vibrational modes calculated with scaled quantum mechanics (SQM) method. 1H and 13C NMR spectra are recorded and the chemical shifts are calculated by using DFT/B3LYP methods with 6-311++G(d,p) basis set. The UV absorption spectrum of the studied compound is recorded in the range of 200–400 nm by dissolved in ethanol. The optimized geometric parameters were compared with experimental data via the X-ray results derived from complexes of this molecule. In addition these, molecular electrostatic potential (MEP), thermodynamic and electronic properties, HOMO–LUMO energies and Mulliken atomic charges, are performed.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide