| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233191 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 13 Pages |
•The compound was characterized by FT-IR, FT-Raman, NMR and UV spectroscopy.•The vibrational frequencies, chemical shifts and electronic absorption wavelengths were calculated by DFT.•Density of state (TDOS, PDOS, and OPDOS) plots for 3,5-Difluoroaniline were analyzed.•NLO properties and NBO analysis were investigated.
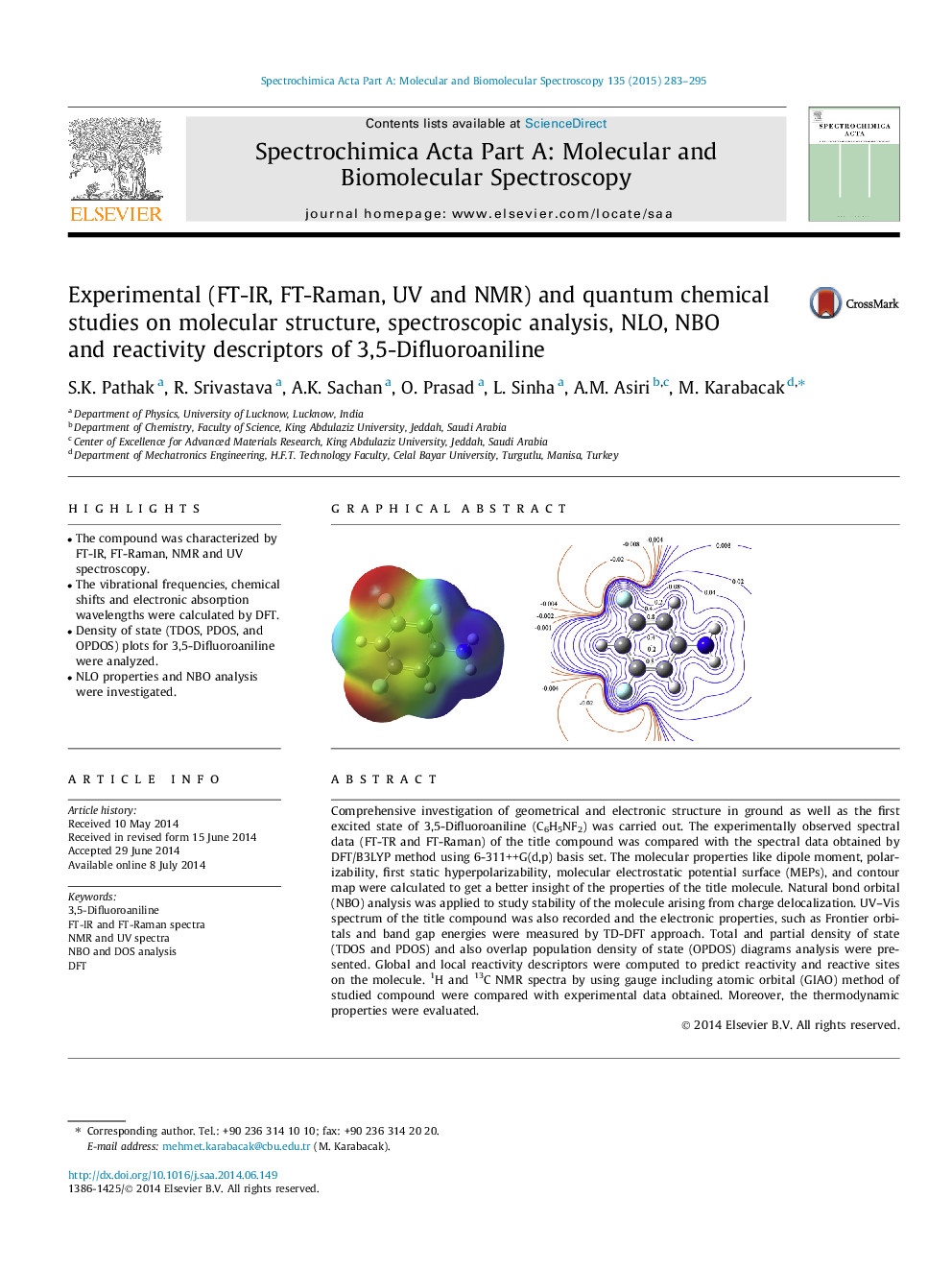
Comprehensive investigation of geometrical and electronic structure in ground as well as the first excited state of 3,5-Difluoroaniline (C6H5NF2) was carried out. The experimentally observed spectral data (FT-TR and FT-Raman) of the title compound was compared with the spectral data obtained by DFT/B3LYP method using 6-311++G(d,p) basis set. The molecular properties like dipole moment, polarizability, first static hyperpolarizability, molecular electrostatic potential surface (MEPs), and contour map were calculated to get a better insight of the properties of the title molecule. Natural bond orbital (NBO) analysis was applied to study stability of the molecule arising from charge delocalization. UV–Vis spectrum of the title compound was also recorded and the electronic properties, such as Frontier orbitals and band gap energies were measured by TD-DFT approach. Total and partial density of state (TDOS and PDOS) and also overlap population density of state (OPDOS) diagrams analysis were presented. Global and local reactivity descriptors were computed to predict reactivity and reactive sites on the molecule. 1H and 13C NMR spectra by using gauge including atomic orbital (GIAO) method of studied compound were compared with experimental data obtained. Moreover, the thermodynamic properties were evaluated.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide