| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233196 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 7 Pages |
•Single step synthesis of surface functionalized fluorescent CdS quantum dots.•Selective fluorescence responses for Cu2+ and Hg2+ ions.•Selective fluorescent switching with Cu2+.•Enhanced fluorescence (4-fold) of CdS quantum dots by reducing pH from 10.0 to 2.0.•Selective sensing of Cu2+ and Hg2+ ions in real water samples.
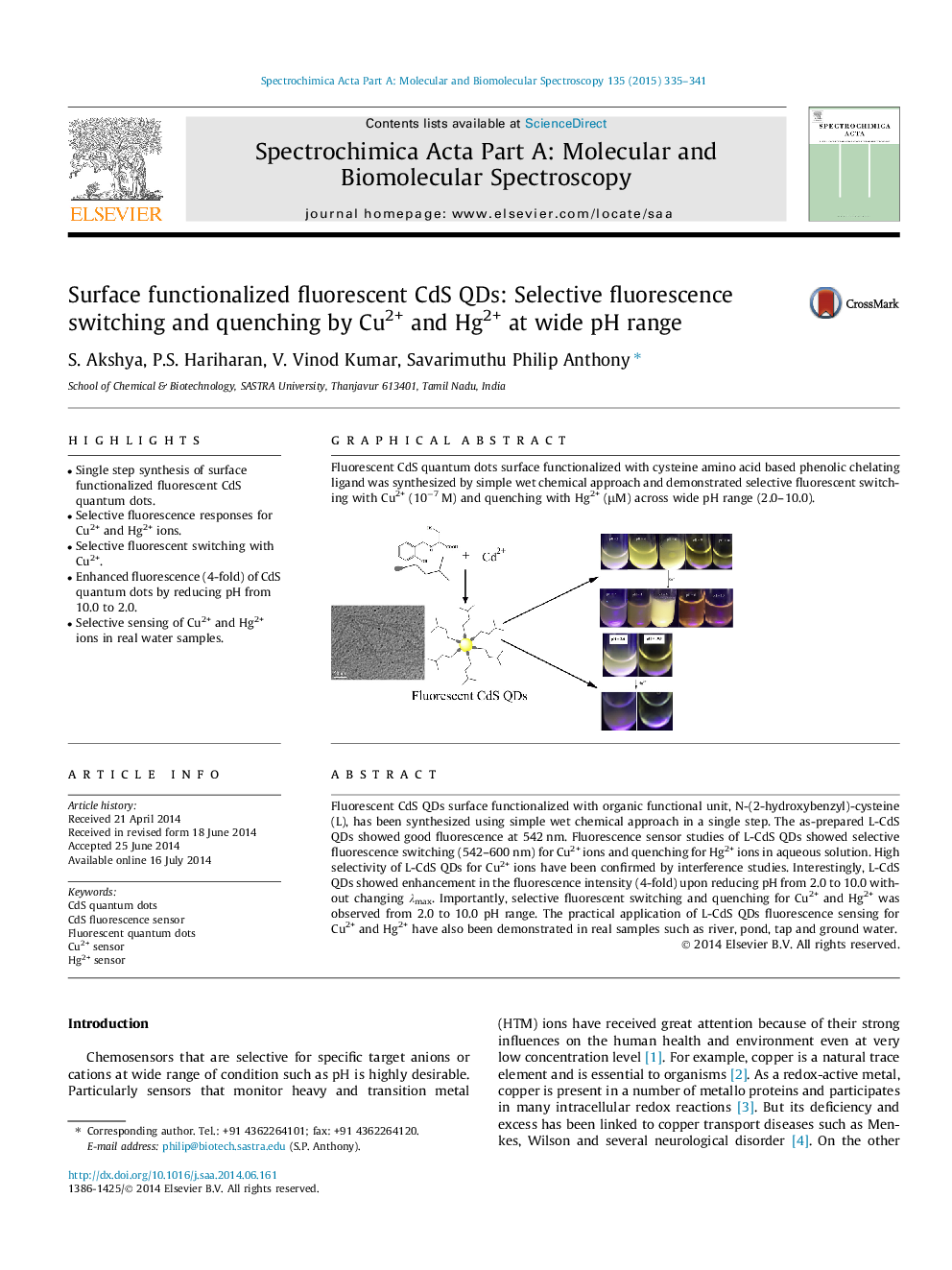
Fluorescent CdS QDs surface functionalized with organic functional unit, N-(2-hydroxybenzyl)-cysteine (L), has been synthesized using simple wet chemical approach in a single step. The as-prepared L-CdS QDs showed good fluorescence at 542 nm. Fluorescence sensor studies of L-CdS QDs showed selective fluorescence switching (542–600 nm) for Cu2+ ions and quenching for Hg2+ ions in aqueous solution. High selectivity of L-CdS QDs for Cu2+ ions have been confirmed by interference studies. Interestingly, L-CdS QDs showed enhancement in the fluorescence intensity (4-fold) upon reducing pH from 2.0 to 10.0 without changing λmax. Importantly, selective fluorescent switching and quenching for Cu2+ and Hg2+ was observed from 2.0 to 10.0 pH range. The practical application of L-CdS QDs fluorescence sensing for Cu2+ and Hg2+ have also been demonstrated in real samples such as river, pond, tap and ground water.
Graphical abstractFluorescent CdS quantum dots surface functionalized with cysteine amino acid based phenolic chelating ligand was synthesized by simple wet chemical approach and demonstrated selective fluorescent switching with Cu2+ (10−7 M) and quenching with Hg2+ (μM) across wide pH range (2.0–10.0).Figure optionsDownload full-size imageDownload as PowerPoint slide