| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233202 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 7 Pages |
•We designed and synthesized two novel carbazole-based dye molecules.•The dye D4 containing thiophene group showed better photoelectronic and photovoltaic properties than D3.•The two dyes could be applied as sensitizers in nano-TiO2 DSSCs.
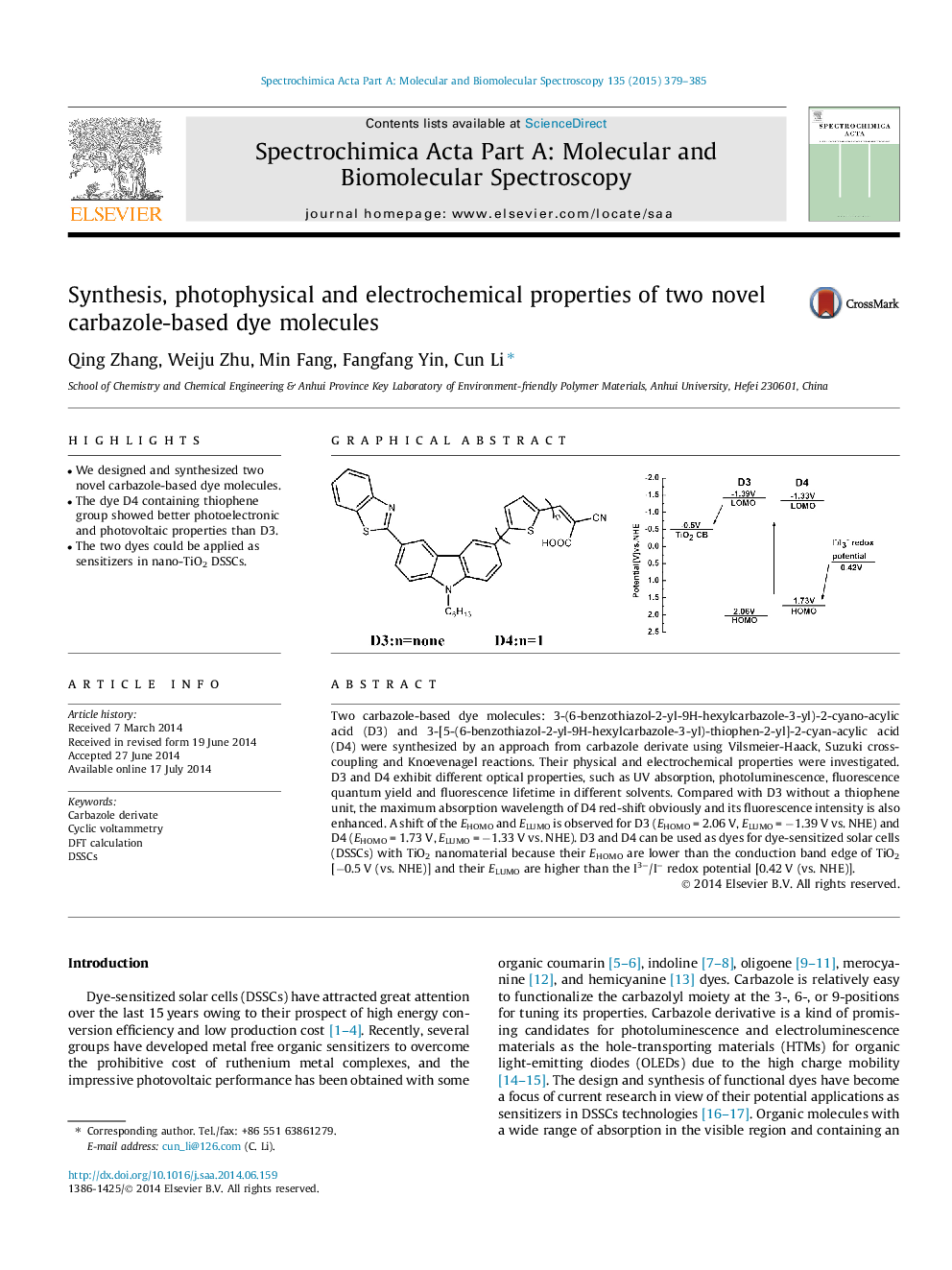
Two carbazole-based dye molecules: 3-(6-benzothiazol-2-yl-9H-hexylcarbazole-3-yl)-2-cyano-acylic acid (D3) and 3-[5-(6-benzothiazol-2-yl-9H-hexylcarbazole-3-yl)-thiophen-2-yl]-2-cyan-acylic acid (D4) were synthesized by an approach from carbazole derivate using Vilsmeier-Haack, Suzuki cross-coupling and Knoevenagel reactions. Their physical and electrochemical properties were investigated. D3 and D4 exhibit different optical properties, such as UV absorption, photoluminescence, fluorescence quantum yield and fluorescence lifetime in different solvents. Compared with D3 without a thiophene unit, the maximum absorption wavelength of D4 red-shift obviously and its fluorescence intensity is also enhanced. A shift of the EHOMO and ELUMO is observed for D3 (EHOMO = 2.06 V, ELUMO = −1.39 V vs. NHE) and D4 (EHOMO = 1.73 V, ELUMO = −1.33 V vs. NHE). D3 and D4 can be used as dyes for dye-sensitized solar cells (DSSCs) with TiO2 nanomaterial because their EHOMO are lower than the conduction band edge of TiO2 [−0.5 V (vs. NHE)] and their ELUMO are higher than the I3−/I− redox potential [0.42 V (vs. NHE)].
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide