| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233203 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 12 Pages |
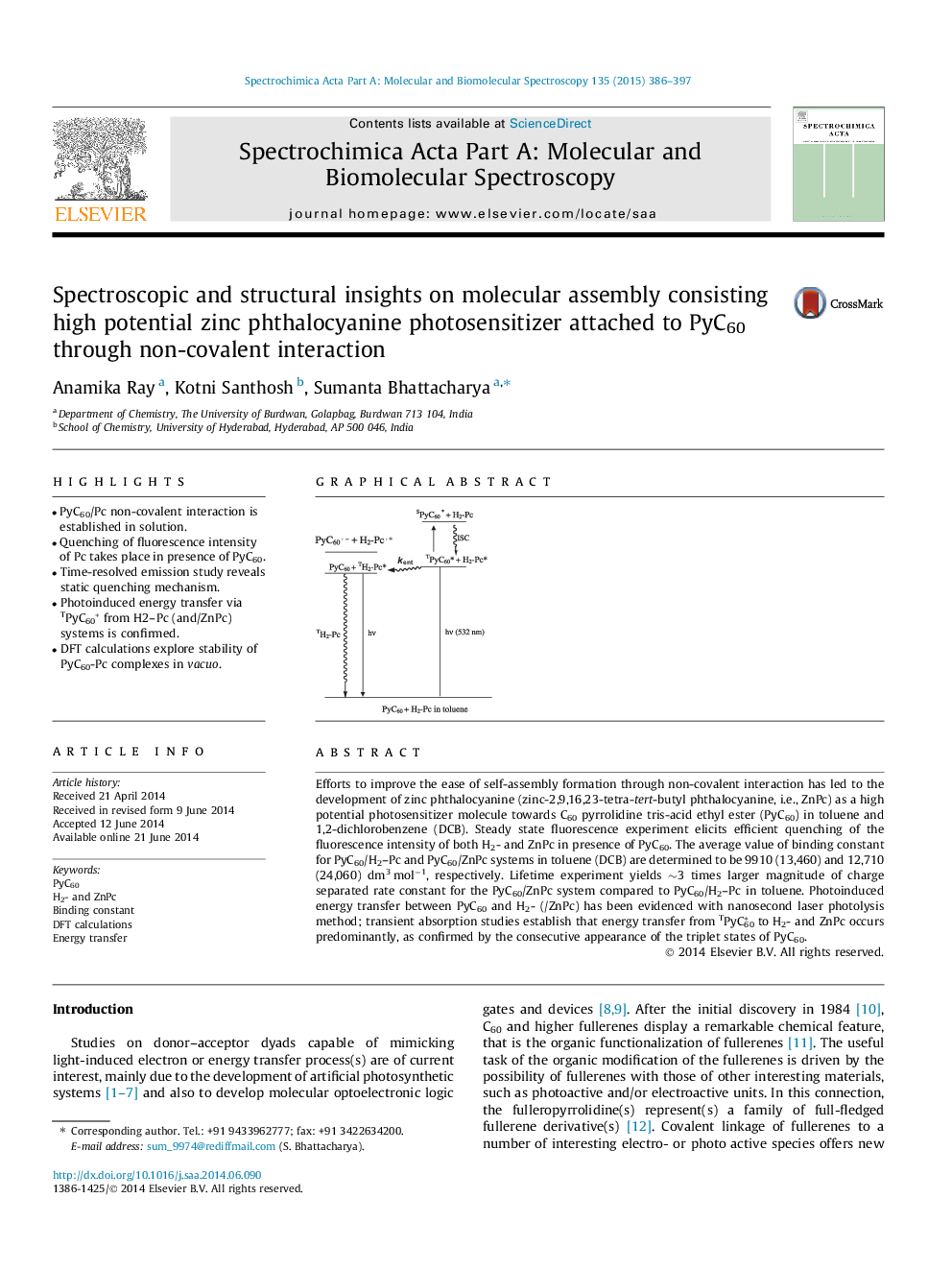
•PyC60/Pc non-covalent interaction is established in solution.•Quenching of fluorescence intensity of Pc takes place in presence of PyC60.•Time-resolved emission study reveals static quenching mechanism.•Photoinduced energy transfer via TPyC60* from H2–Pc (and/ZnPc) systems is confirmed.•DFT calculations explore stability of PyC60-Pc complexes in vacuo.
Efforts to improve the ease of self-assembly formation through non-covalent interaction has led to the development of zinc phthalocyanine (zinc-2,9,16,23-tetra-tert-butyl phthalocyanine, i.e., ZnPc) as a high potential photosensitizer molecule towards C60 pyrrolidine tris-acid ethyl ester (PyC60) in toluene and 1,2-dichlorobenzene (DCB). Steady state fluorescence experiment elicits efficient quenching of the fluorescence intensity of both H2- and ZnPc in presence of PyC60. The average value of binding constant for PyC60/H2–Pc and PyC60/ZnPc systems in toluene (DCB) are determined to be 9910 (13,460) and 12,710 (24,060) dm3 mol−1, respectively. Lifetime experiment yields ∼3 times larger magnitude of charge separated rate constant for the PyC60/ZnPc system compared to PyC60/H2–Pc in toluene. Photoinduced energy transfer between PyC60 and H2- (/ZnPc) has been evidenced with nanosecond laser photolysis method; transient absorption studies establish that energy transfer from TPyC60∗ to H2- and ZnPc occurs predominantly, as confirmed by the consecutive appearance of the triplet states of PyC60.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide