| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233209 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 12 Pages |
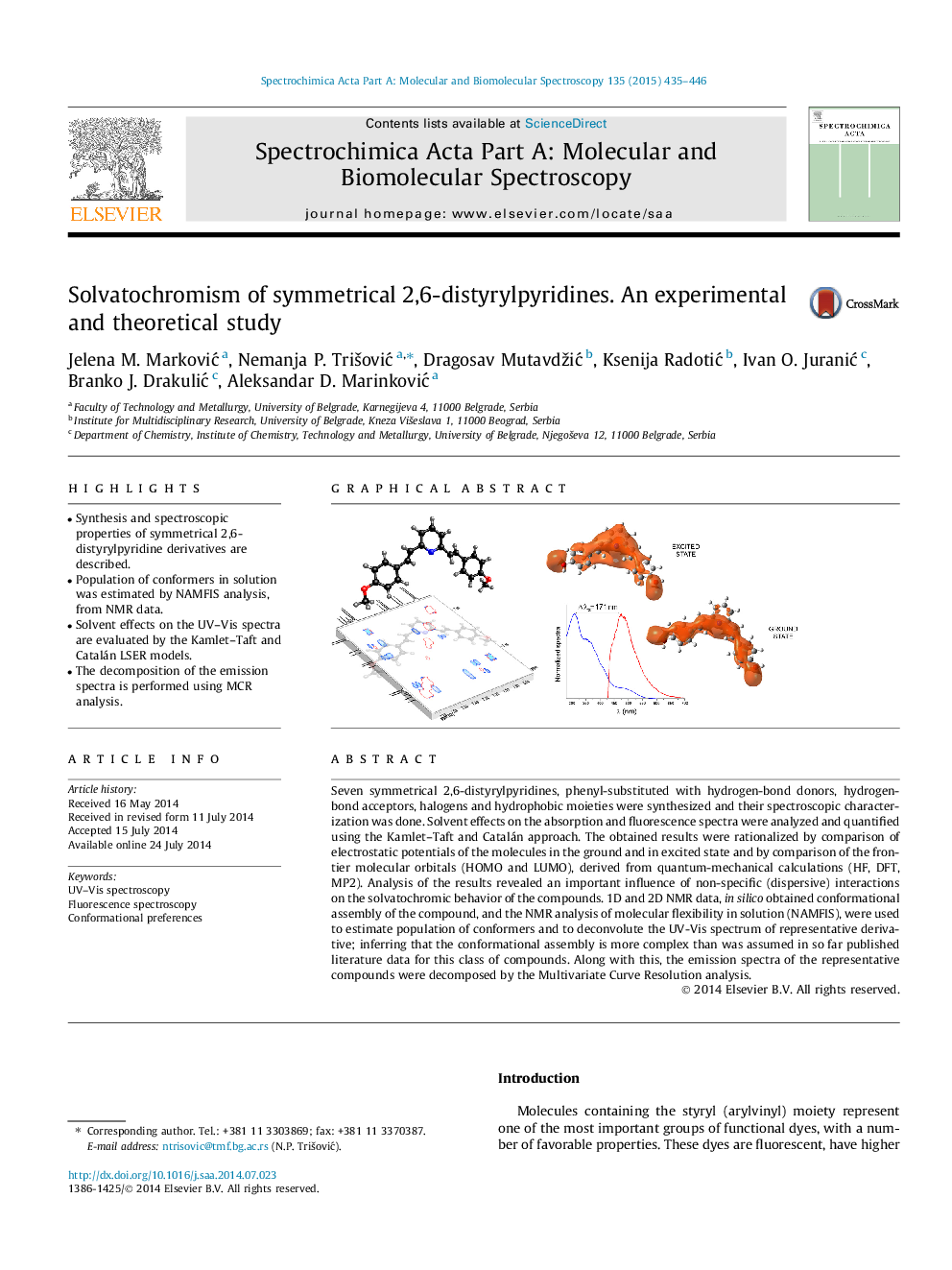
•Synthesis and spectroscopic properties of symmetrical 2,6-distyrylpyridine derivatives are described.•Population of conformers in solution was estimated by NAMFIS analysis, from NMR data.•Solvent effects on the UV–Vis spectra are evaluated by the Kamlet–Taft and Catalán LSER models.•The decomposition of the emission spectra is performed using MCR analysis.
Seven symmetrical 2,6-distyrylpyridines, phenyl-substituted with hydrogen-bond donors, hydrogen-bond acceptors, halogens and hydrophobic moieties were synthesized and their spectroscopic characterization was done. Solvent effects on the absorption and fluorescence spectra were analyzed and quantified using the Kamlet–Taft and Catalán approach. The obtained results were rationalized by comparison of electrostatic potentials of the molecules in the ground and in excited state and by comparison of the frontier molecular orbitals (HOMO and LUMO), derived from quantum-mechanical calculations (HF, DFT, MP2). Analysis of the results revealed an important influence of non-specific (dispersive) interactions on the solvatochromic behavior of the compounds. 1D and 2D NMR data, in silico obtained conformational assembly of the compound, and the NMR analysis of molecular flexibility in solution (NAMFIS), were used to estimate population of conformers and to deconvolute the UV-Vis spectrum of representative derivative; inferring that the conformational assembly is more complex than was assumed in so far published literature data for this class of compounds. Along with this, the emission spectra of the representative compounds were decomposed by the Multivariate Curve Resolution analysis.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide