| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233210 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 10 Pages |
•ZnTPP was spectrophotometrically titrated with a diverse suite of organophosphates (OP).•Polarizability of OP substituents determines extent of ZnTPP λSoret shift.•Aλsor-b for the OP bound ZnTPP varied with size and aromatic content of substituents.•Ab-initio ZnTPP–OP energies reveal steric effects on binding.
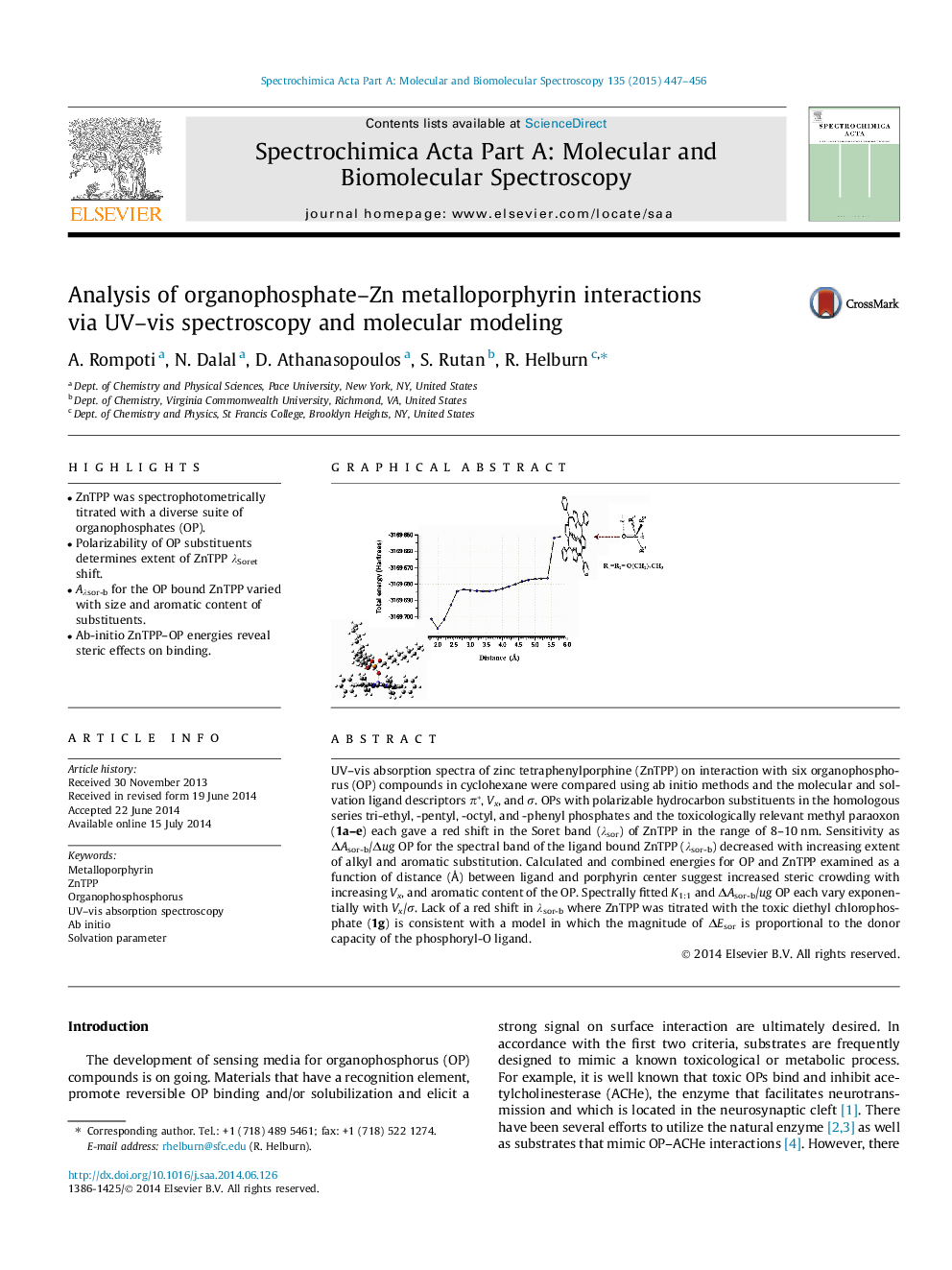
UV–vis absorption spectra of zinc tetraphenylporphine (ZnTPP) on interaction with six organophosphorus (OP) compounds in cyclohexane were compared using ab initio methods and the molecular and solvation ligand descriptors π*, Vx, and σ. OPs with polarizable hydrocarbon substituents in the homologous series tri-ethyl, -pentyl, -octyl, and -phenyl phosphates and the toxicologically relevant methyl paraoxon (1a–e) each gave a red shift in the Soret band (λsor) of ZnTPP in the range of 8–10 nm. Sensitivity as ΔAsor-b/Δug OP for the spectral band of the ligand bound ZnTPP (λsor-b) decreased with increasing extent of alkyl and aromatic substitution. Calculated and combined energies for OP and ZnTPP examined as a function of distance (Å) between ligand and porphyrin center suggest increased steric crowding with increasing Vx, and aromatic content of the OP. Spectrally fitted K1:1 and ΔAsor-b/ug OP each vary exponentially with Vx/σ. Lack of a red shift in λsor-b where ZnTPP was titrated with the toxic diethyl chlorophosphate (1g) is consistent with a model in which the magnitude of ΔEsor is proportional to the donor capacity of the phosphoryl-O ligand.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide