| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233212 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 6 Pages |
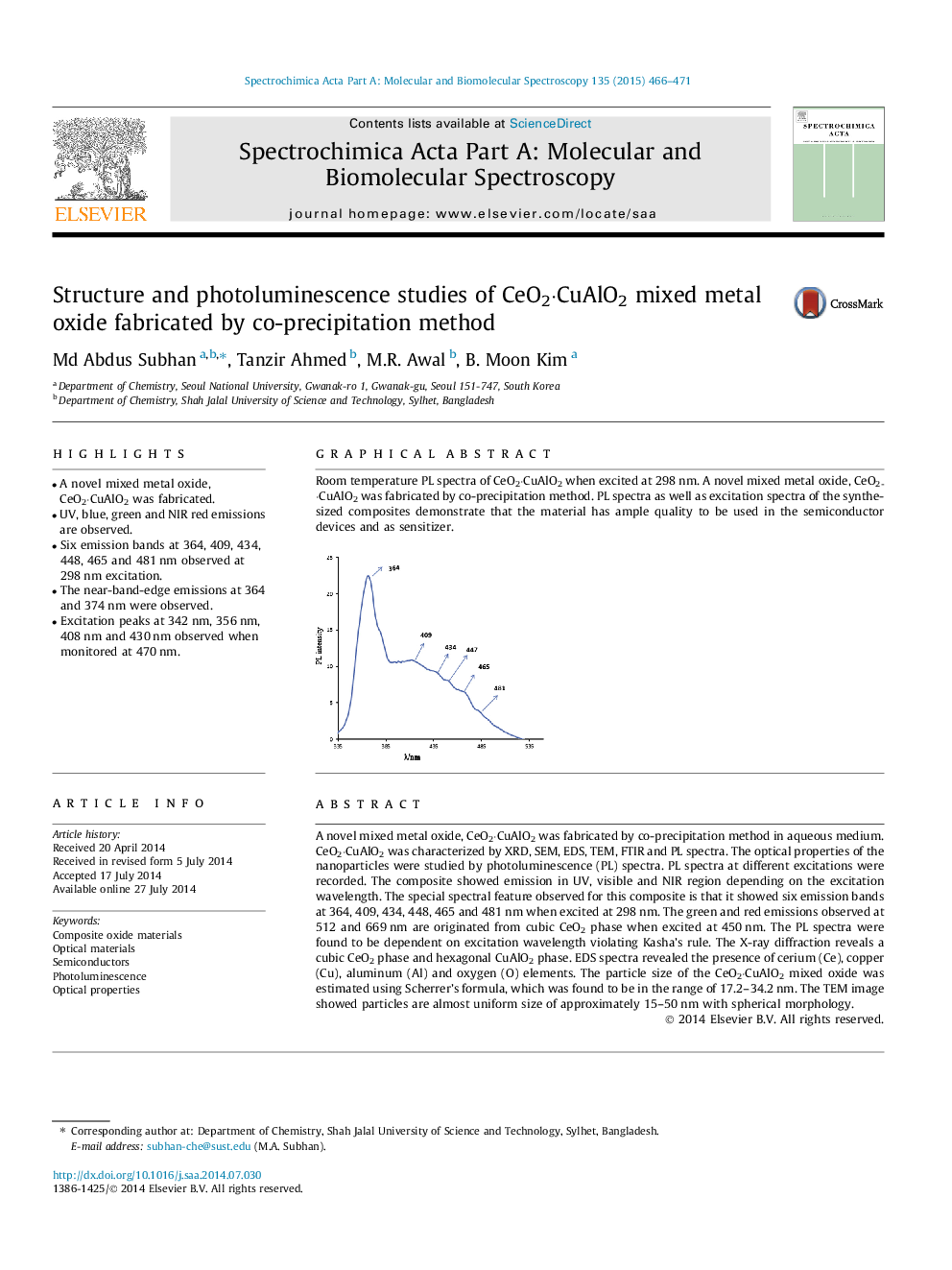
•A novel mixed metal oxide, CeO2·CuAlO2 was fabricated.•UV, blue, green and NIR red emissions are observed.•Six emission bands at 364, 409, 434, 448, 465 and 481 nm observed at 298 nm excitation.•The near-band-edge emissions at 364 and 374 nm were observed.•Excitation peaks at 342 nm, 356 nm, 408 nm and 430 nm observed when monitored at 470 nm.
A novel mixed metal oxide, CeO2·CuAlO2 was fabricated by co-precipitation method in aqueous medium. CeO2·CuAlO2 was characterized by XRD, SEM, EDS, TEM, FTIR and PL spectra. The optical properties of the nanoparticles were studied by photoluminescence (PL) spectra. PL spectra at different excitations were recorded. The composite showed emission in UV, visible and NIR region depending on the excitation wavelength. The special spectral feature observed for this composite is that it showed six emission bands at 364, 409, 434, 448, 465 and 481 nm when excited at 298 nm. The green and red emissions observed at 512 and 669 nm are originated from cubic CeO2 phase when excited at 450 nm. The PL spectra were found to be dependent on excitation wavelength violating Kasha’s rule. The X-ray diffraction reveals a cubic CeO2 phase and hexagonal CuAlO2 phase. EDS spectra revealed the presence of cerium (Ce), copper (Cu), aluminum (Al) and oxygen (O) elements. The particle size of the CeO2·CuAlO2 mixed oxide was estimated using Scherrer’s formula, which was found to be in the range of 17.2–34.2 nm. The TEM image showed particles are almost uniform size of approximately 15–50 nm with spherical morphology.
Graphical abstractRoom temperature PL spectra of CeO2·CuAlO2 when excited at 298 nm. A novel mixed metal oxide, CeO2·CuAlO2 was fabricated by co-precipitation method. PL spectra as well as excitation spectra of the synthesized composites demonstrate that the material has ample quality to be used in the semiconductor devices and as sensitizer.Figure optionsDownload full-size imageDownload as PowerPoint slide