| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233221 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 4 Pages |
•PEG coated Fe3O4 nanoparticles synthesized by chemical co-precipitation method.•The PEG coated Fe3O4 nanoparticles has promising for biomedical applications.•The PEG weight increases also the crystalline size and magnetic values decreases.
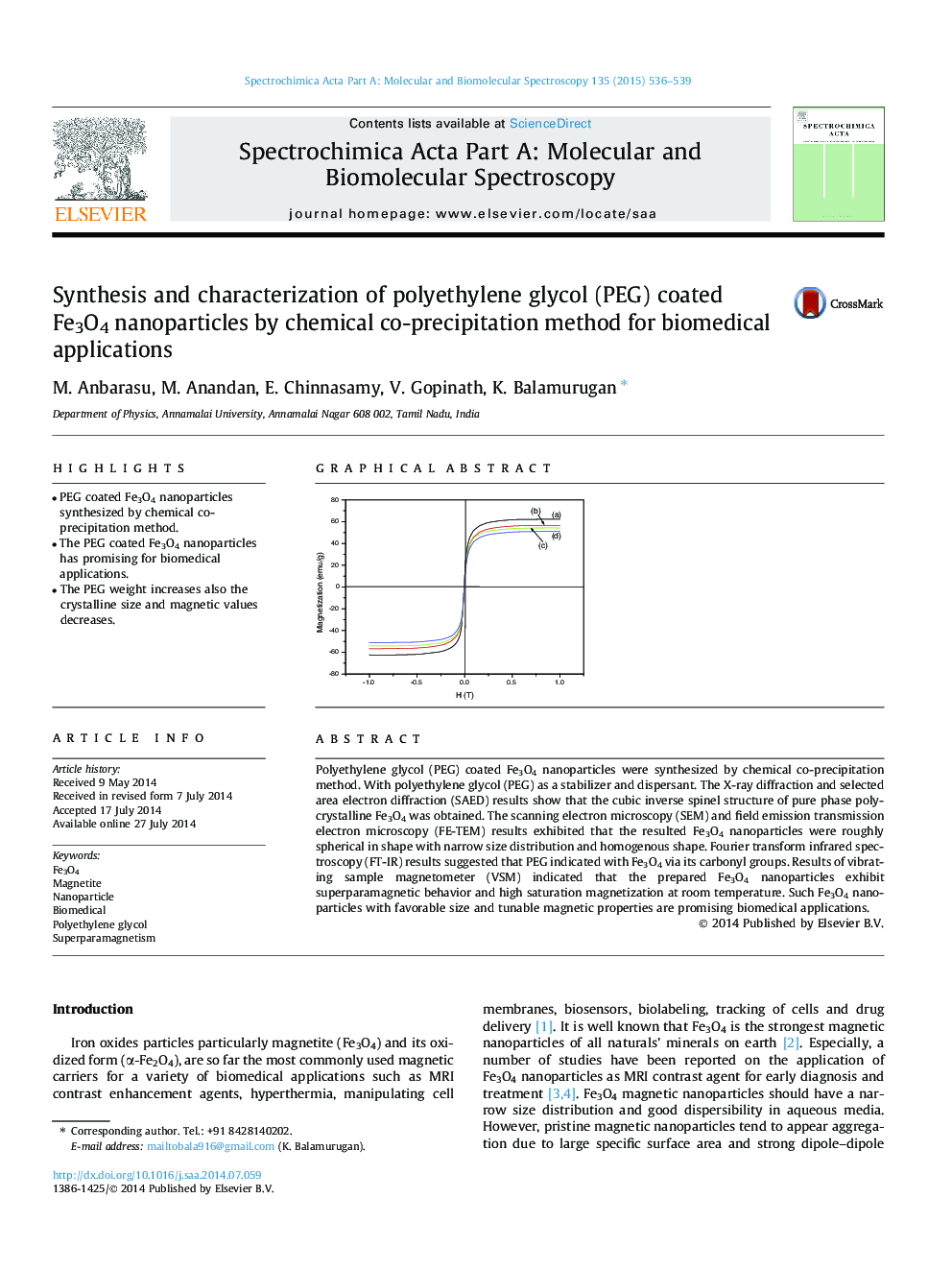
Polyethylene glycol (PEG) coated Fe3O4 nanoparticles were synthesized by chemical co-precipitation method. With polyethylene glycol (PEG) as a stabilizer and dispersant. The X-ray diffraction and selected area electron diffraction (SAED) results show that the cubic inverse spinel structure of pure phase polycrystalline Fe3O4 was obtained. The scanning electron microscopy (SEM) and field emission transmission electron microscopy (FE-TEM) results exhibited that the resulted Fe3O4 nanoparticles were roughly spherical in shape with narrow size distribution and homogenous shape. Fourier transform infrared spectroscopy (FT-IR) results suggested that PEG indicated with Fe3O4 via its carbonyl groups. Results of vibrating sample magnetometer (VSM) indicated that the prepared Fe3O4 nanoparticles exhibit superparamagnetic behavior and high saturation magnetization at room temperature. Such Fe3O4 nanoparticles with favorable size and tunable magnetic properties are promising biomedical applications.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide