| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233222 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 11 Pages |
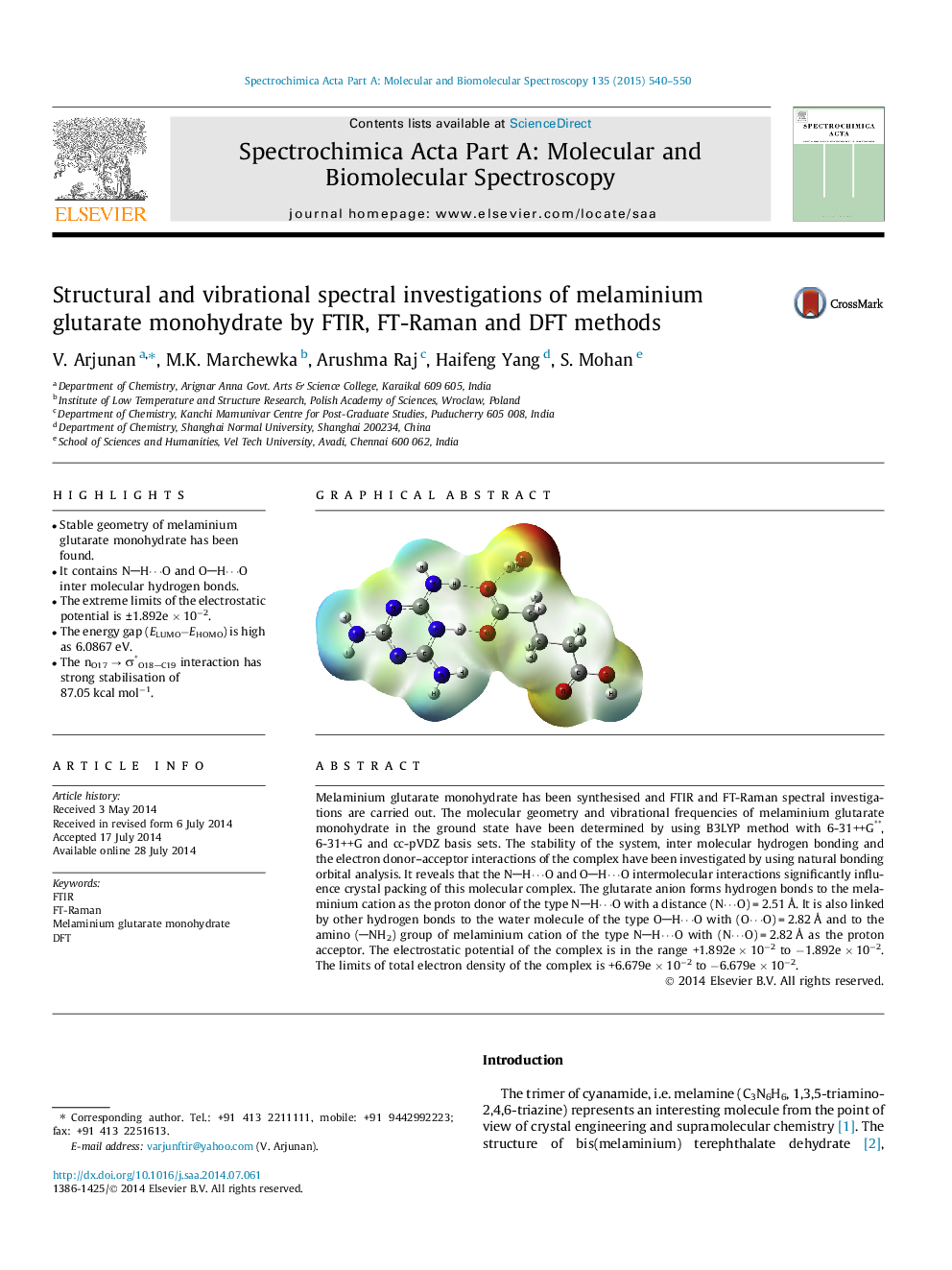
•Stable geometry of melaminium glutarate monohydrate has been found.•It contains NH⋯O and OH⋯O inter molecular hydrogen bonds.•The extreme limits of the electrostatic potential is ±1.892e × 10−2.•The energy gap (ELUMO−EHOMO) is high as 6.0867 eV.•The nO17 → σ*O18C19 interaction has strong stabilisation of 87.05 kcal mol−1.
Melaminium glutarate monohydrate has been synthesised and FTIR and FT-Raman spectral investigations are carried out. The molecular geometry and vibrational frequencies of melaminium glutarate monohydrate in the ground state have been determined by using B3LYP method with 6-31++G**, 6-31++G and cc-pVDZ basis sets. The stability of the system, inter molecular hydrogen bonding and the electron donor–acceptor interactions of the complex have been investigated by using natural bonding orbital analysis. It reveals that the NH⋯O and OH⋯O intermolecular interactions significantly influence crystal packing of this molecular complex. The glutarate anion forms hydrogen bonds to the melaminium cation as the proton donor of the type NH⋯O with a distance (N⋯O) = 2.51 Å. It is also linked by other hydrogen bonds to the water molecule of the type OH⋯O with (O⋯O) = 2.82 Å and to the amino (NH2) group of melaminium cation of the type NH⋯O with (N⋯O) = 2.82 Å as the proton acceptor. The electrostatic potential of the complex is in the range +1.892e × 10−2 to −1.892e × 10−2. The limits of total electron density of the complex is +6.679e × 10−2 to −6.679e × 10−2.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide