| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233224 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 8 Pages |
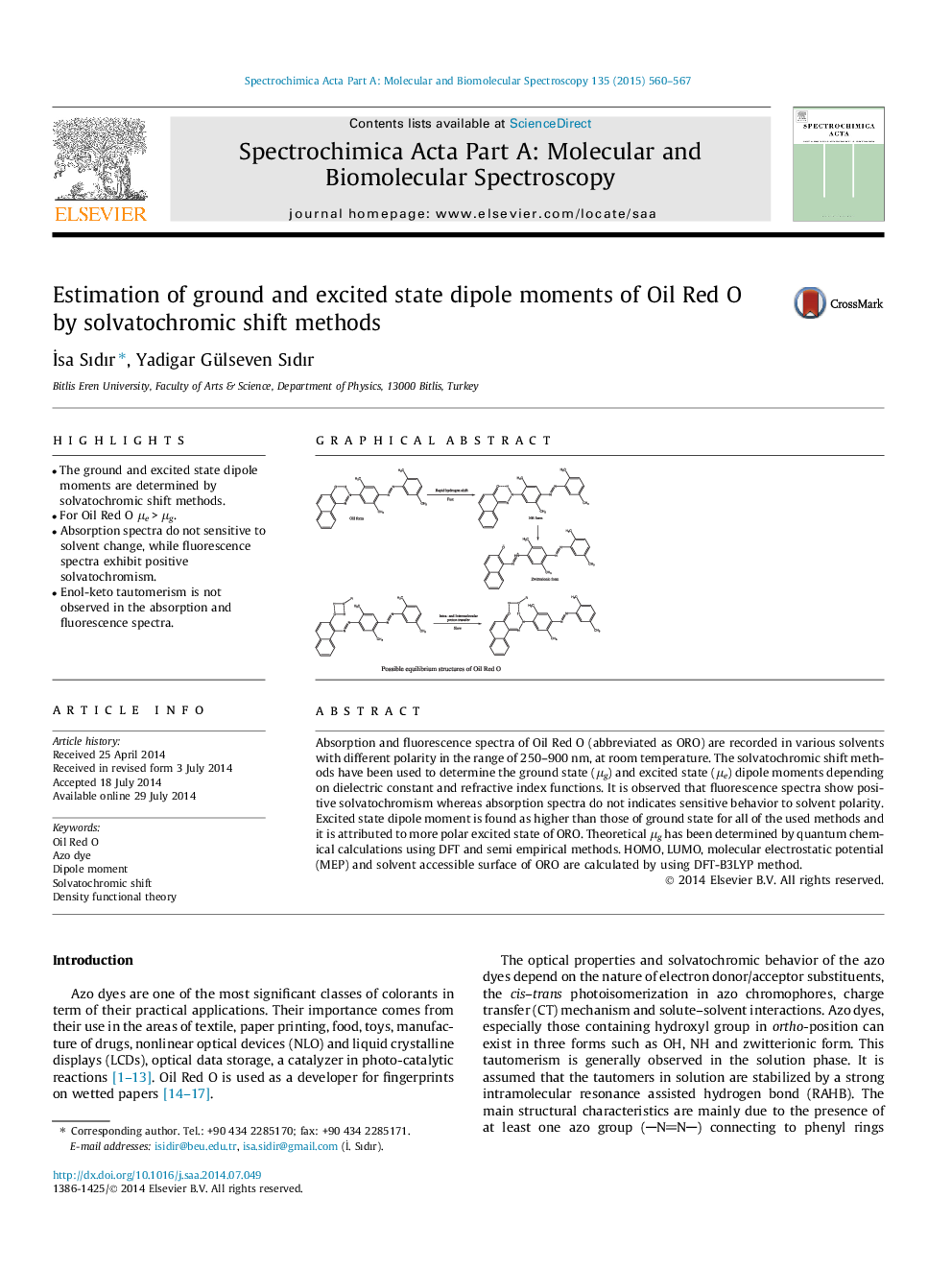
•The ground and excited state dipole moments are determined by solvatochromic shift methods.•For Oil Red O μe > μg.•Absorption spectra do not sensitive to solvent change, while fluorescence spectra exhibit positive solvatochromism.•Enol-keto tautomerism is not observed in the absorption and fluorescence spectra.
Absorption and fluorescence spectra of Oil Red O (abbreviated as ORO) are recorded in various solvents with different polarity in the range of 250–900 nm, at room temperature. The solvatochromic shift methods have been used to determine the ground state (μg) and excited state (μe) dipole moments depending on dielectric constant and refractive index functions. It is observed that fluorescence spectra show positive solvatochromism whereas absorption spectra do not indicates sensitive behavior to solvent polarity. Excited state dipole moment is found as higher than those of ground state for all of the used methods and it is attributed to more polar excited state of ORO. Theoretical μg has been determined by quantum chemical calculations using DFT and semi empirical methods. HOMO, LUMO, molecular electrostatic potential (MEP) and solvent accessible surface of ORO are calculated by using DFT-B3LYP method.
Graphical abstractPossible equilibrium structures of Oil Red O.Figure optionsDownload full-size imageDownload as PowerPoint slide