| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233415 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 6 Pages |
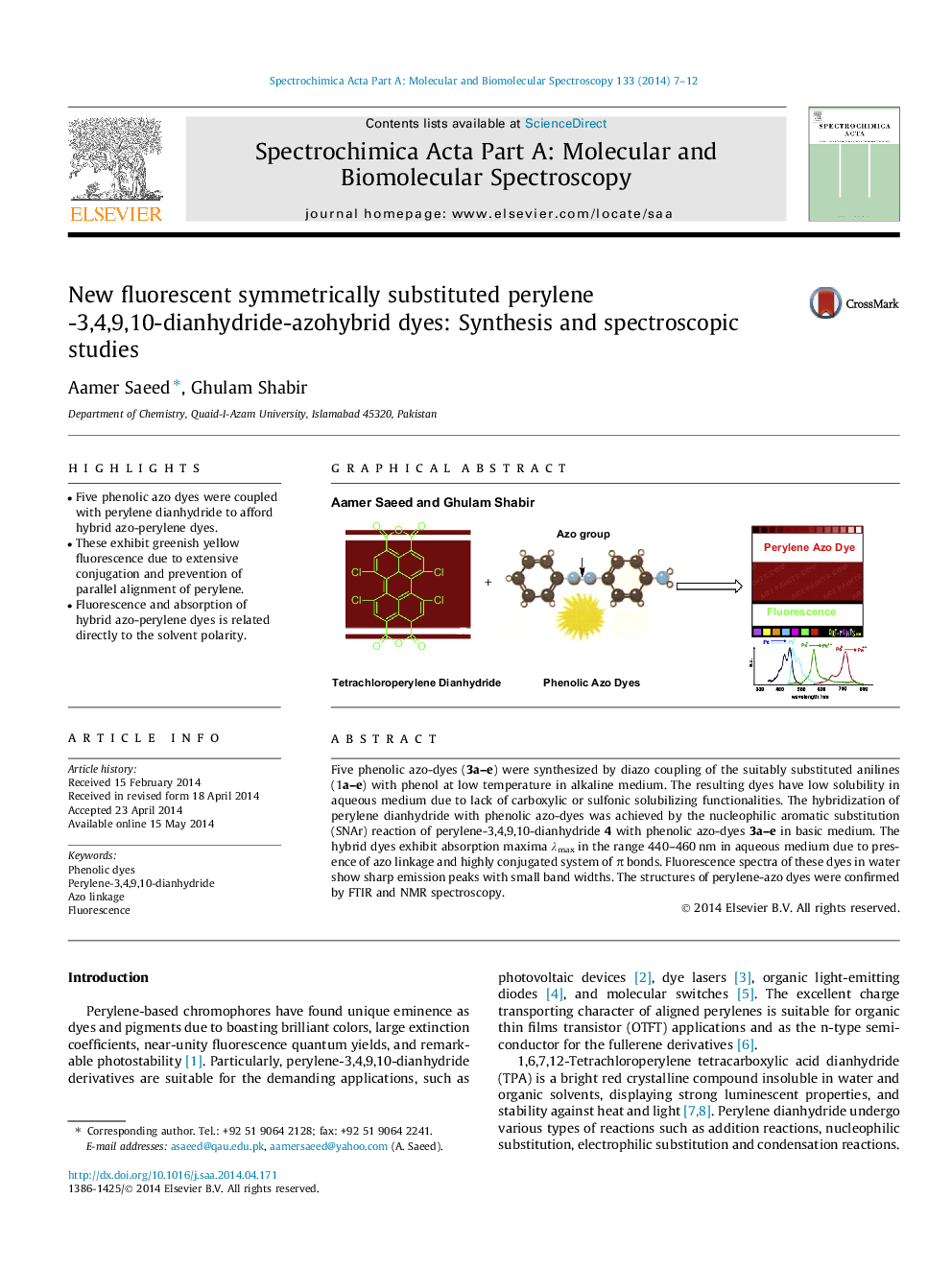
•Five phenolic azo dyes were coupled with perylene dianhydride to afford hybrid azo-perylene dyes.•These exhibit greenish yellow fluorescence due to extensive conjugation and prevention of parallel alignment of perylene.•Fluorescence and absorption of hybrid azo-perylene dyes is related directly to the solvent polarity.
Five phenolic azo-dyes (3a–e) were synthesized by diazo coupling of the suitably substituted anilines (1a–e) with phenol at low temperature in alkaline medium. The resulting dyes have low solubility in aqueous medium due to lack of carboxylic or sulfonic solubilizing functionalities. The hybridization of perylene dianhydride with phenolic azo-dyes was achieved by the nucleophilic aromatic substitution (SNAr) reaction of perylene-3,4,9,10-dianhydride 4 with phenolic azo-dyes 3a–e in basic medium. The hybrid dyes exhibit absorption maxima λmax in the range 440–460 nm in aqueous medium due to presence of azo linkage and highly conjugated system of π bonds. Fluorescence spectra of these dyes in water show sharp emission peaks with small band widths. The structures of perylene-azo dyes were confirmed by FTIR and NMR spectroscopy.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide