| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233445 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 6 Pages |
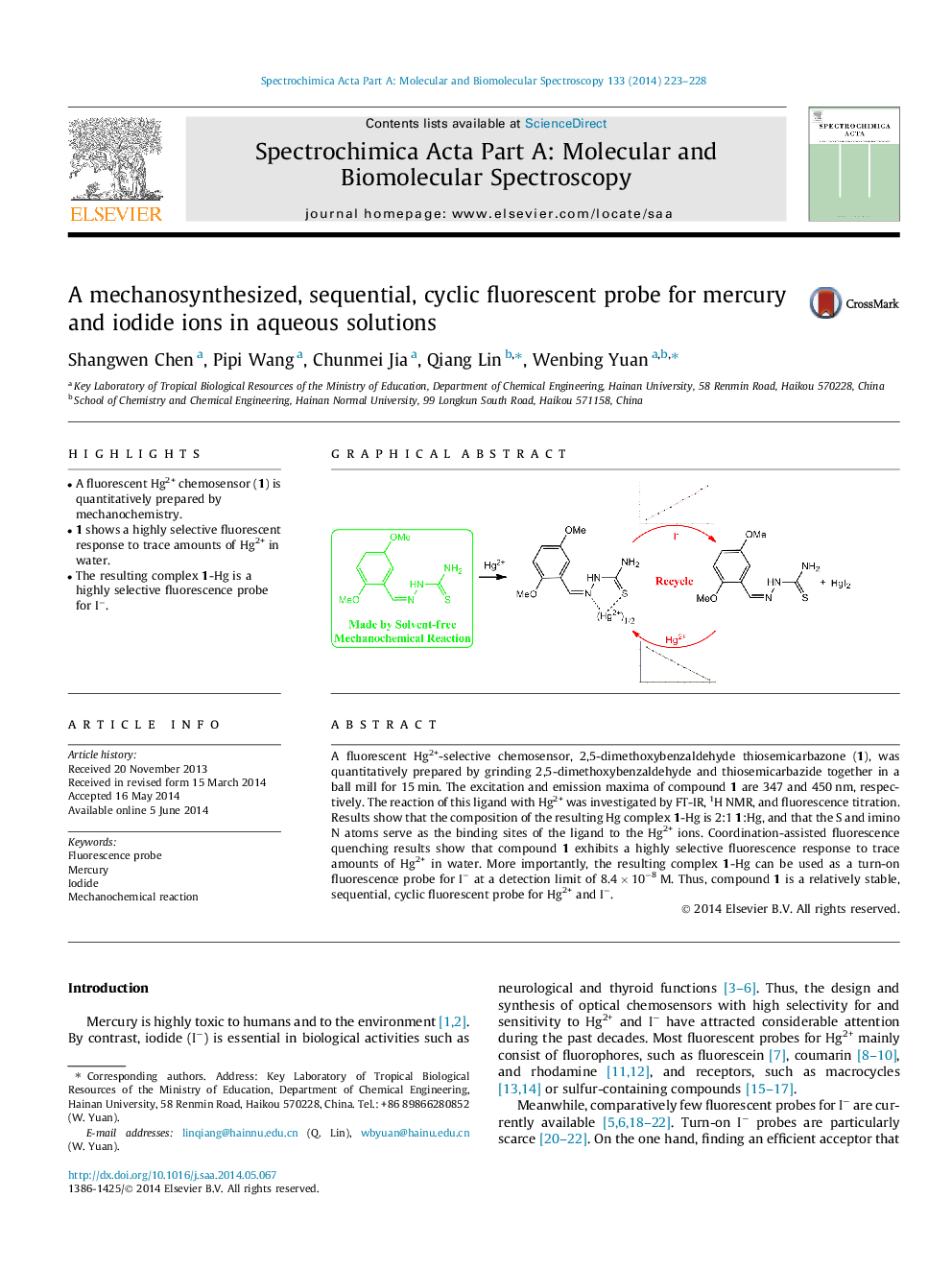
•A fluorescent Hg2+ chemosensor (1) is quantitatively prepared by mechanochemistry.•1 shows a highly selective fluorescent response to trace amounts of Hg2+ in water.•The resulting complex 1-Hg is a highly selective fluorescence probe for I−.
A fluorescent Hg2+-selective chemosensor, 2,5-dimethoxybenzaldehyde thiosemicarbazone (1), was quantitatively prepared by grinding 2,5-dimethoxybenzaldehyde and thiosemicarbazide together in a ball mill for 15 min. The excitation and emission maxima of compound 1 are 347 and 450 nm, respectively. The reaction of this ligand with Hg2+ was investigated by FT-IR, 1H NMR, and fluorescence titration. Results show that the composition of the resulting Hg complex 1-Hg is 2:1 1:Hg, and that the S and imino N atoms serve as the binding sites of the ligand to the Hg2+ ions. Coordination-assisted fluorescence quenching results show that compound 1 exhibits a highly selective fluorescence response to trace amounts of Hg2+ in water. More importantly, the resulting complex 1-Hg can be used as a turn-on fluorescence probe for I− at a detection limit of 8.4 × 10−8 M. Thus, compound 1 is a relatively stable, sequential, cyclic fluorescent probe for Hg2+ and I−.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide