| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233448 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 9 Pages |
•Raman bands of CH and CSC stretching modes of DMSO were studied in polar solvents.•Experimental results have been compared with the theoretically calculated results.•Variation of bandwidth and peak frequency with solvent concentration was discussed.•Vibrational and reorientation relaxation times in binary mixtures have been studied.•Solvent molecules produce a hindrance to both the relaxation times.
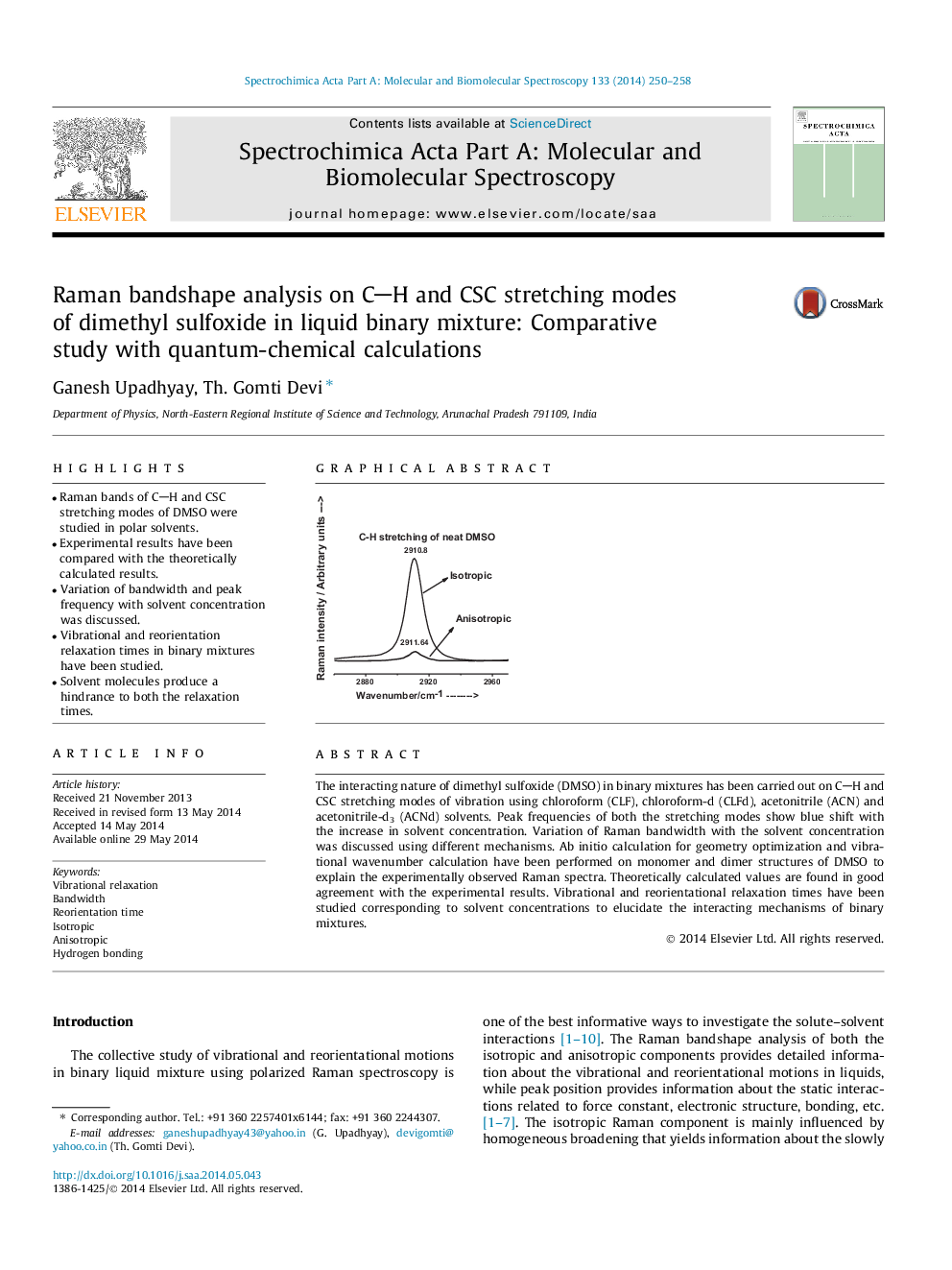
The interacting nature of dimethyl sulfoxide (DMSO) in binary mixtures has been carried out on CH and CSC stretching modes of vibration using chloroform (CLF), chloroform-d (CLFd), acetonitrile (ACN) and acetonitrile-d3 (ACNd) solvents. Peak frequencies of both the stretching modes show blue shift with the increase in solvent concentration. Variation of Raman bandwidth with the solvent concentration was discussed using different mechanisms. Ab initio calculation for geometry optimization and vibrational wavenumber calculation have been performed on monomer and dimer structures of DMSO to explain the experimentally observed Raman spectra. Theoretically calculated values are found in good agreement with the experimental results. Vibrational and reorientational relaxation times have been studied corresponding to solvent concentrations to elucidate the interacting mechanisms of binary mixtures.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide