| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233458 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 5 Pages |
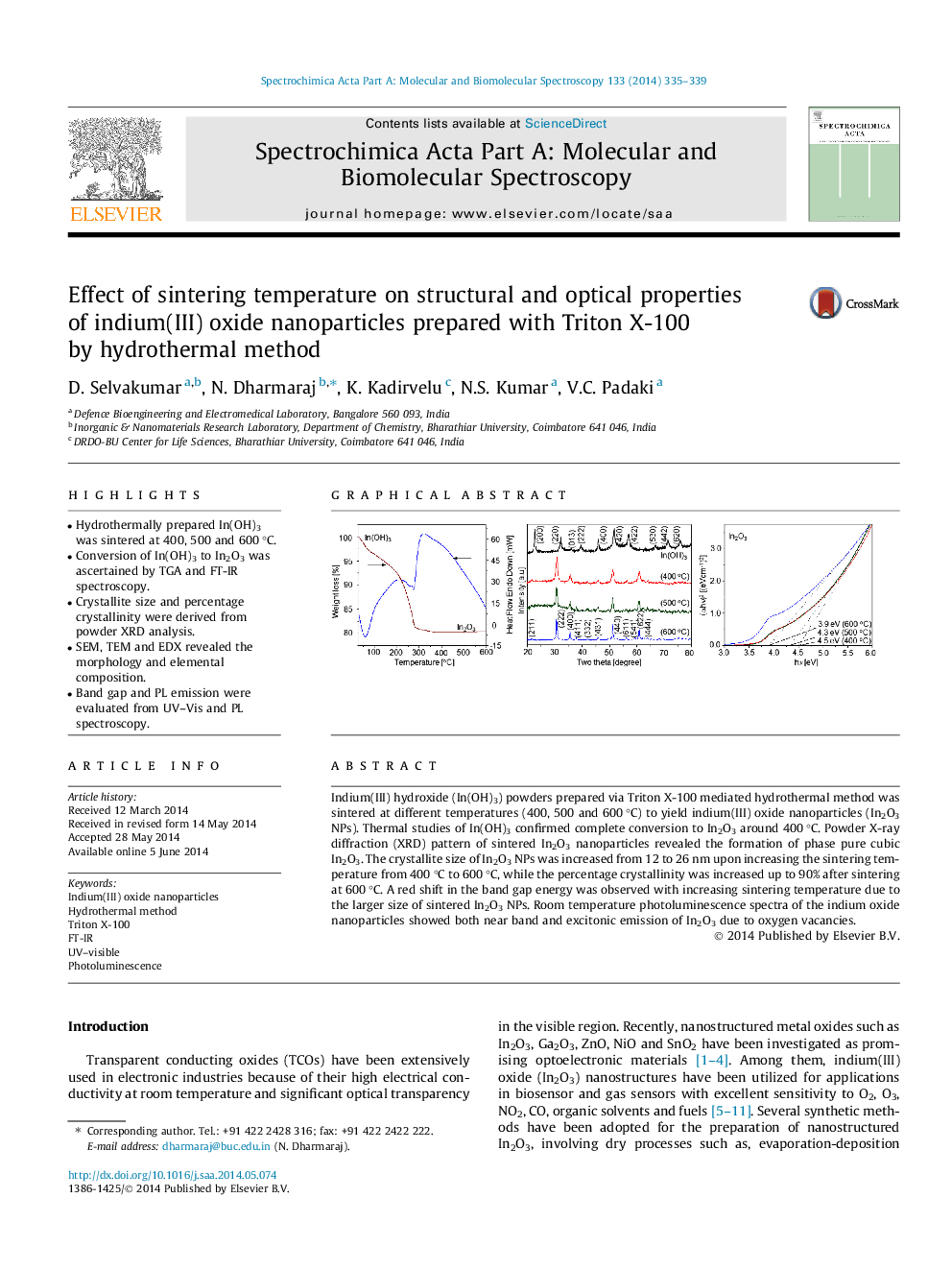
•Hydrothermally prepared In(OH)3 was sintered at 400, 500 and 600 °C.•Conversion of In(OH)3 to In2O3 was ascertained by TGA and FT-IR spectroscopy.•Crystallite size and percentage crystallinity were derived from powder XRD analysis.•SEM, TEM and EDX revealed the morphology and elemental composition.•Band gap and PL emission were evaluated from UV–Vis and PL spectroscopy.
Indium(III) hydroxide (In(OH)3) powders prepared via Triton X-100 mediated hydrothermal method was sintered at different temperatures (400, 500 and 600 °C) to yield indium(III) oxide nanoparticles (In2O3 NPs). Thermal studies of In(OH)3 confirmed complete conversion to In2O3 around 400 °C. Powder X-ray diffraction (XRD) pattern of sintered In2O3 nanoparticles revealed the formation of phase pure cubic In2O3. The crystallite size of In2O3 NPs was increased from 12 to 26 nm upon increasing the sintering temperature from 400 °C to 600 °C, while the percentage crystallinity was increased up to 90% after sintering at 600 °C. A red shift in the band gap energy was observed with increasing sintering temperature due to the larger size of sintered In2O3 NPs. Room temperature photoluminescence spectra of the indium oxide nanoparticles showed both near band and excitonic emission of In2O3 due to oxygen vacancies.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide