| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233472 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 6 Pages |
•The Ce3+/Tb3+ co-doped MyGdFx system samples have been synthesized.•The final samples present different crystalline phases.•Ce3+ and Tb3+ illustrate different optical properties.•The lifetime of Tb3+5D4 → 7F5 decreases with the annealing temperature increasing.•The lifetime of Tb3+5D4 → 7F5 decreases with the increase of the KF concentration.
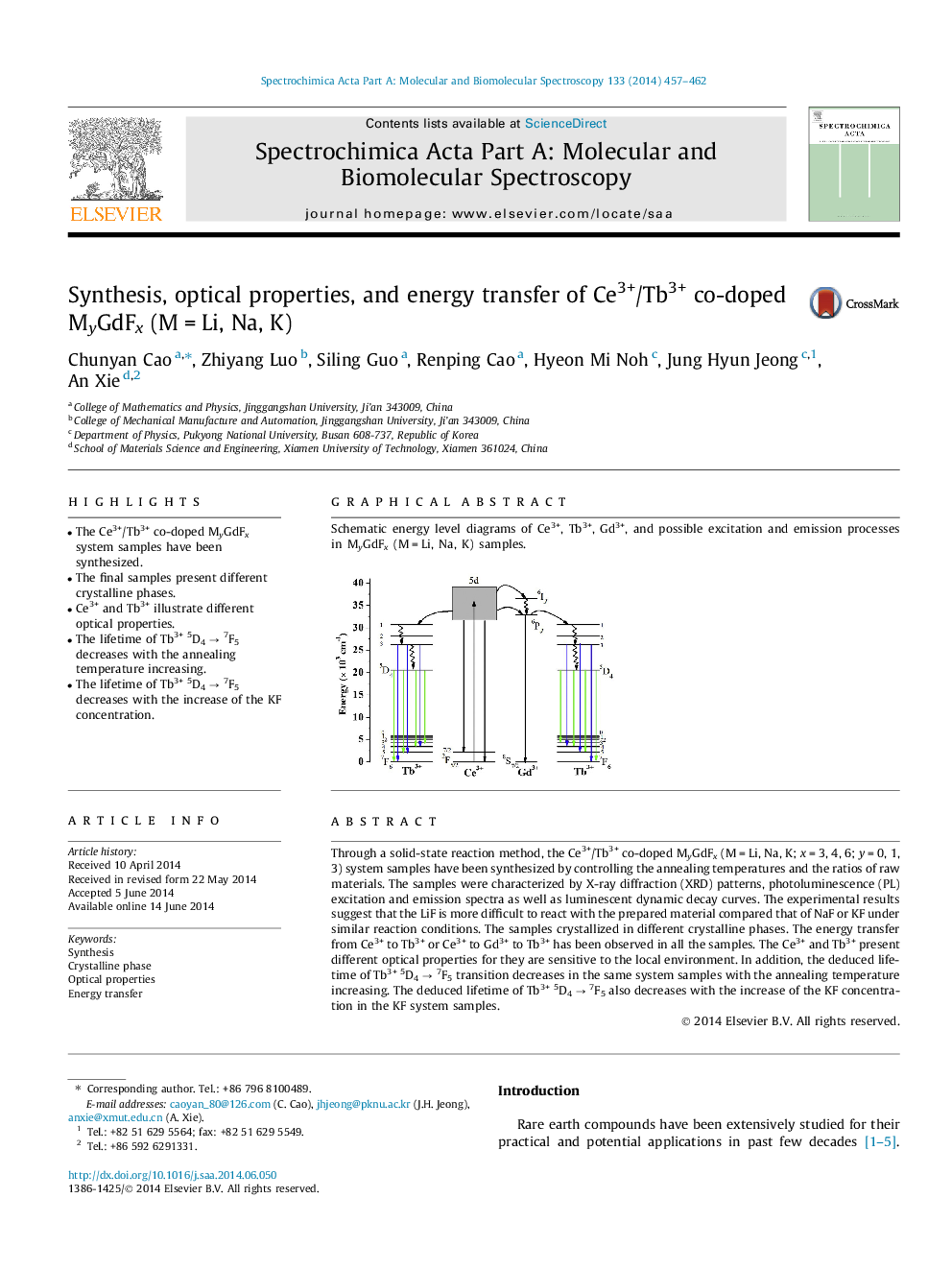
Through a solid-state reaction method, the Ce3+/Tb3+ co-doped MyGdFx (M = Li, Na, K; x = 3, 4, 6; y = 0, 1, 3) system samples have been synthesized by controlling the annealing temperatures and the ratios of raw materials. The samples were characterized by X-ray diffraction (XRD) patterns, photoluminescence (PL) excitation and emission spectra as well as luminescent dynamic decay curves. The experimental results suggest that the LiF is more difficult to react with the prepared material compared that of NaF or KF under similar reaction conditions. The samples crystallized in different crystalline phases. The energy transfer from Ce3+ to Tb3+ or Ce3+ to Gd3+ to Tb3+ has been observed in all the samples. The Ce3+ and Tb3+ present different optical properties for they are sensitive to the local environment. In addition, the deduced lifetime of Tb3+5D4 → 7F5 transition decreases in the same system samples with the annealing temperature increasing. The deduced lifetime of Tb3+5D4 → 7F5 also decreases with the increase of the KF concentration in the KF system samples.
Graphical abstractSchematic energy level diagrams of Ce3+, Tb3+, Gd3+, and possible excitation and emission processes in MyGdFx (M = Li, Na, K) samples.Figure optionsDownload full-size imageDownload as PowerPoint slide