| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233473 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 9 Pages |
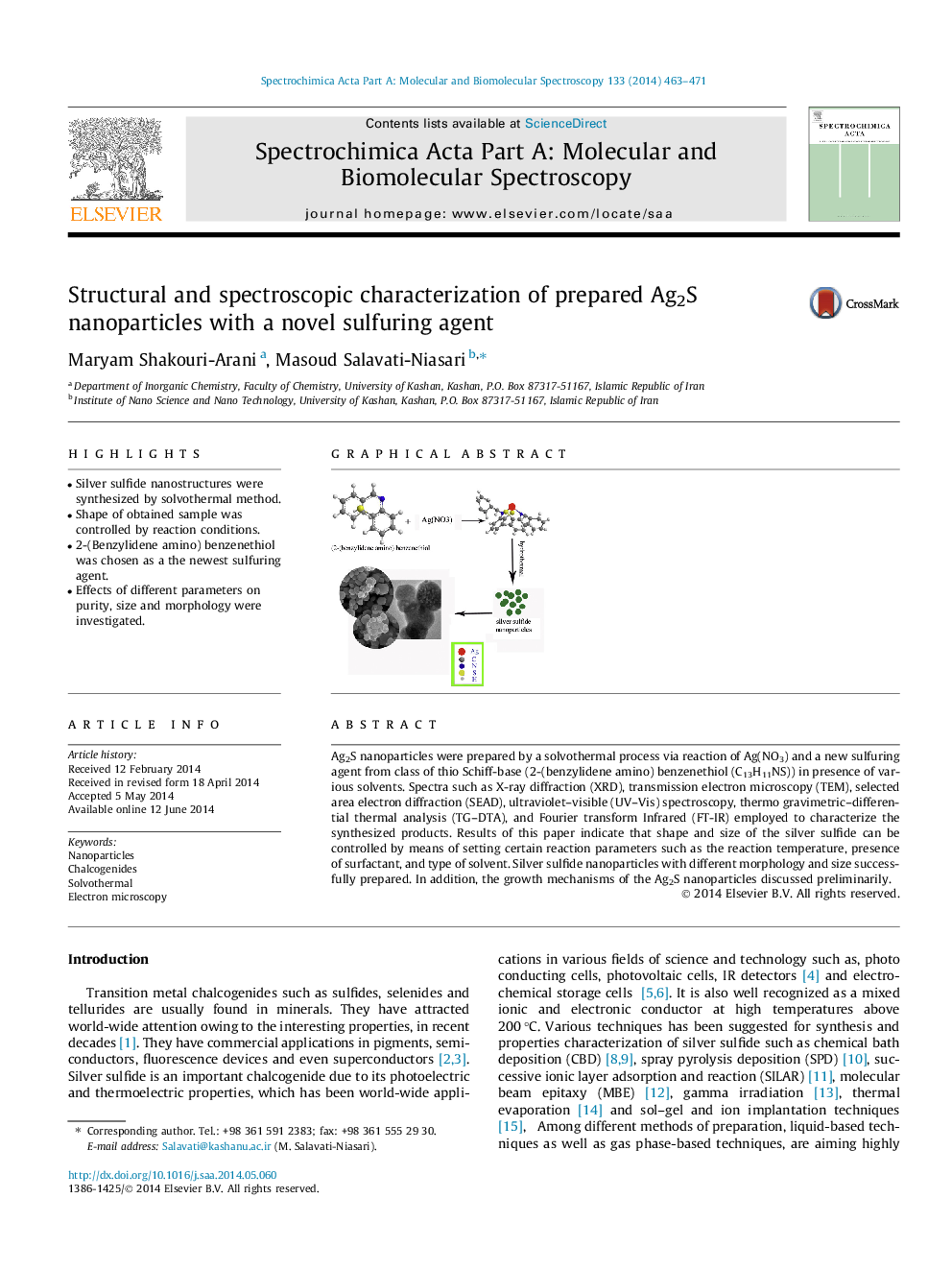
•Silver sulfide nanostructures were synthesized by solvothermal method.•Shape of obtained sample was controlled by reaction conditions.•2-(Benzylidene amino) benzenethiol was chosen as a the newest sulfuring agent.•Effects of different parameters on purity, size and morphology were investigated.
Ag2S nanoparticles were prepared by a solvothermal process via reaction of Ag(NO3) and a new sulfuring agent from class of thio Schiff-base (2-(benzylidene amino) benzenethiol (C13H11NS)) in presence of various solvents. Spectra such as X-ray diffraction (XRD), transmission electron microscopy (TEM), selected area electron diffraction (SEAD), ultraviolet–visible (UV–Vis) spectroscopy, thermo gravimetric–differential thermal analysis (TG–DTA), and Fourier transform Infrared (FT-IR) employed to characterize the synthesized products. Results of this paper indicate that shape and size of the silver sulfide can be controlled by means of setting certain reaction parameters such as the reaction temperature, presence of surfactant, and type of solvent. Silver sulfide nanoparticles with different morphology and size successfully prepared. In addition, the growth mechanisms of the Ag2S nanoparticles discussed preliminarily.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide