| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233474 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 8 Pages |
•Silver nanoparticles (Ag NPs) have been synthesized by Dirk and Charles method.•Spherical shaped Ag NPs were obtained.•Quenching and binding constants of DMDMAD with Ag NPs were calculated.•Fluorescence quantum yield of DMDMAD with Ag NPs have been calculated.•Energy transfer efficiency and critical transfer distance were calculated.
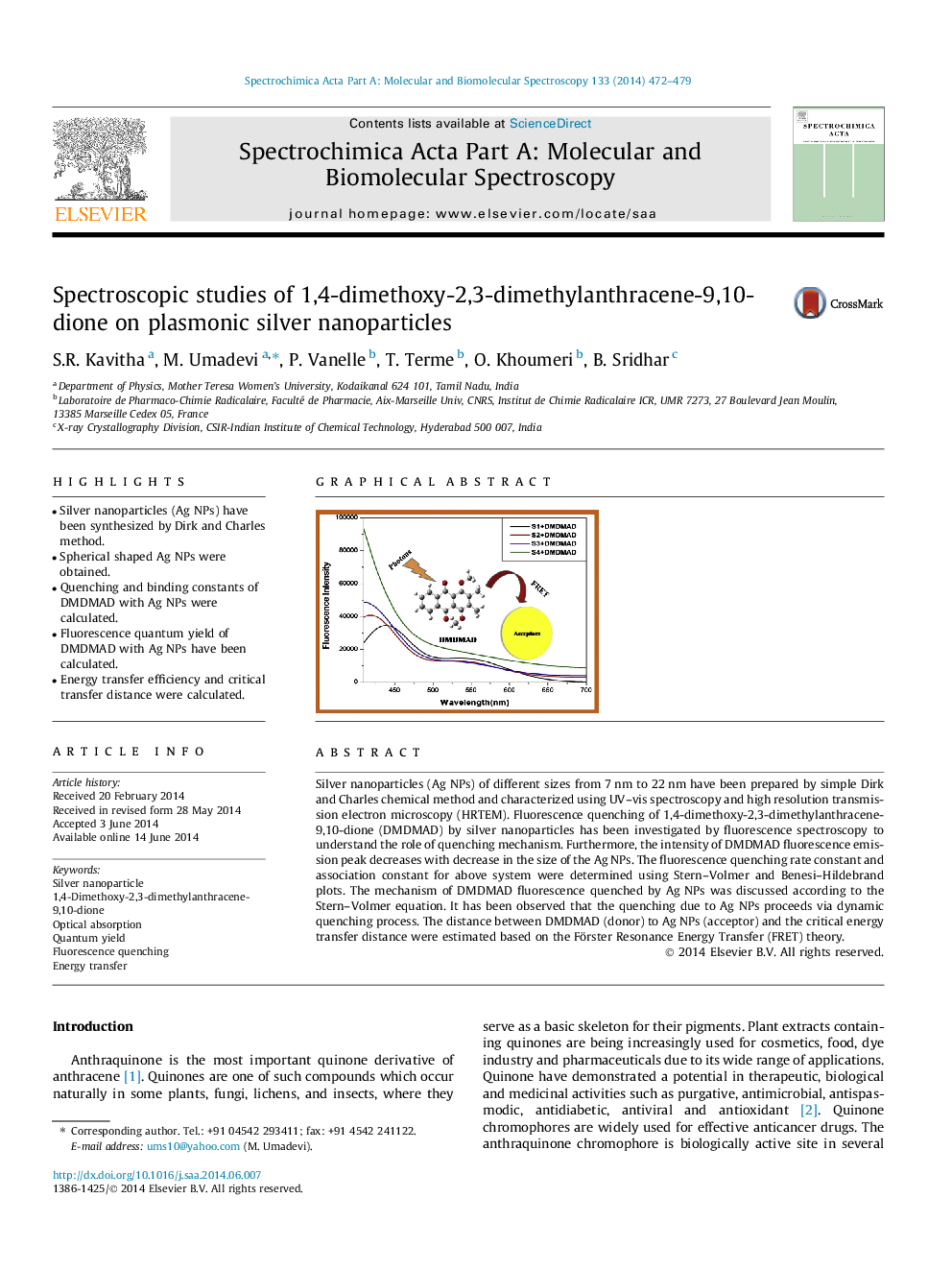
Silver nanoparticles (Ag NPs) of different sizes from 7 nm to 22 nm have been prepared by simple Dirk and Charles chemical method and characterized using UV–vis spectroscopy and high resolution transmission electron microscopy (HRTEM). Fluorescence quenching of 1,4-dimethoxy-2,3-dimethylanthracene-9,10-dione (DMDMAD) by silver nanoparticles has been investigated by fluorescence spectroscopy to understand the role of quenching mechanism. Furthermore, the intensity of DMDMAD fluorescence emission peak decreases with decrease in the size of the Ag NPs. The fluorescence quenching rate constant and association constant for above system were determined using Stern–Volmer and Benesi–Hildebrand plots. The mechanism of DMDMAD fluorescence quenched by Ag NPs was discussed according to the Stern–Volmer equation. It has been observed that the quenching due to Ag NPs proceeds via dynamic quenching process. The distance between DMDMAD (donor) to Ag NPs (acceptor) and the critical energy transfer distance were estimated based on the Förster Resonance Energy Transfer (FRET) theory.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide