| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233486 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 9 Pages |
•Two new dibenzoxanthene compounds were synthesized and characterized.•DNA-binding behaviors were studied by spectroscopic titration, viscosity and photocleavage.•The cytotoxicity of these compounds was evaluated by MTT method.•The apoptosis were investigated by AO/EB staining method.•The cellular uptake, ROS, mitochondrial membrane potential and cell cycle arrest were investigated.
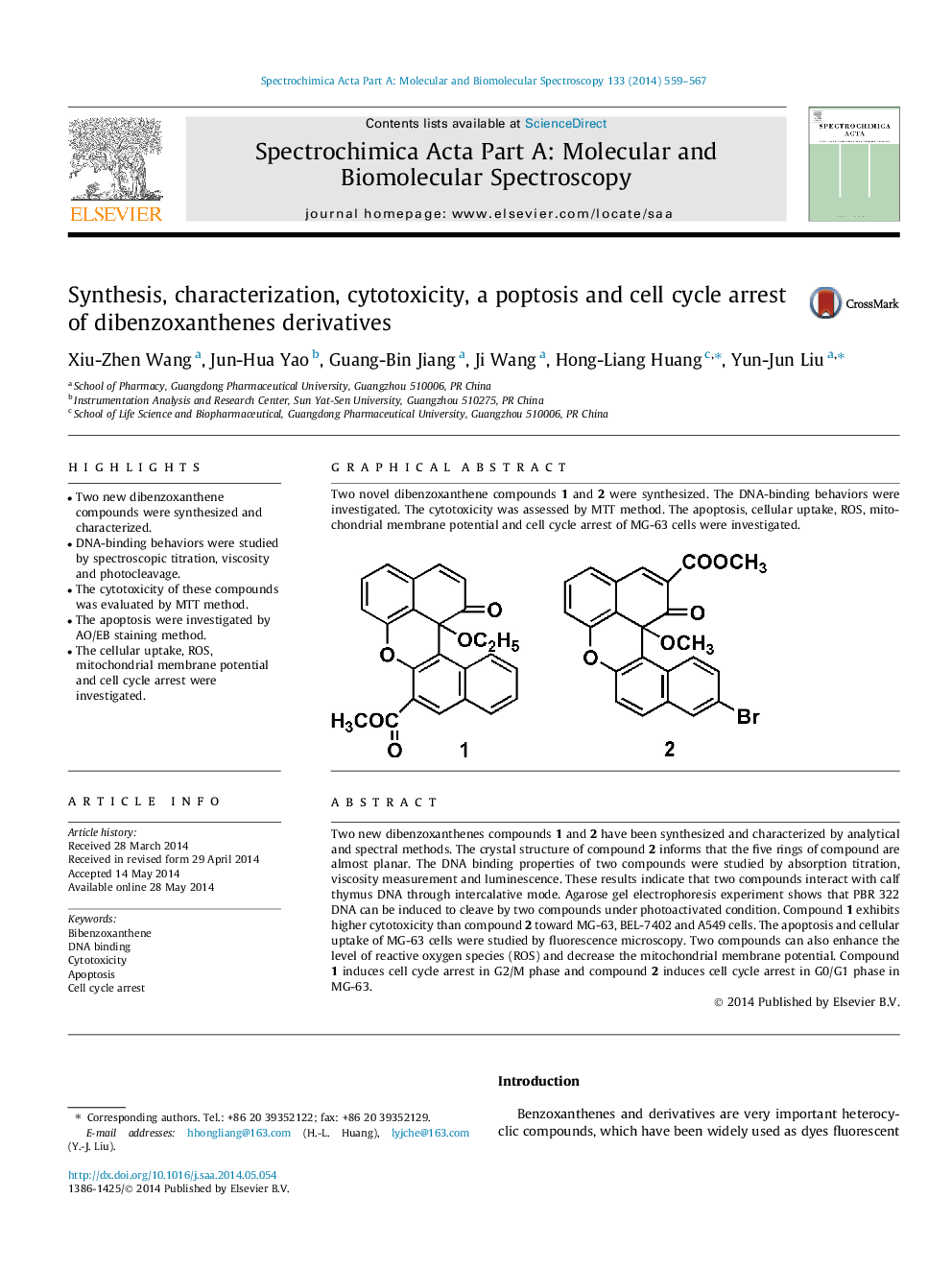
Two new dibenzoxanthenes compounds 1 and 2 have been synthesized and characterized by analytical and spectral methods. The crystal structure of compound 2 informs that the five rings of compound are almost planar. The DNA binding properties of two compounds were studied by absorption titration, viscosity measurement and luminescence. These results indicate that two compounds interact with calf thymus DNA through intercalative mode. Agarose gel electrophoresis experiment shows that PBR 322 DNA can be induced to cleave by two compounds under photoactivated condition. Compound 1 exhibits higher cytotoxicity than compound 2 toward MG-63, BEL-7402 and A549 cells. The apoptosis and cellular uptake of MG-63 cells were studied by fluorescence microscopy. Two compounds can also enhance the level of reactive oxygen species (ROS) and decrease the mitochondrial membrane potential. Compound 1 induces cell cycle arrest in G2/M phase and compound 2 induces cell cycle arrest in G0/G1 phase in MG-63.
Graphical abstractTwo novel dibenzoxanthene compounds 1 and 2 were synthesized. The DNA-binding behaviors were investigated. The cytotoxicity was assessed by MTT method. The apoptosis, cellular uptake, ROS, mitochondrial membrane potential and cell cycle arrest of MG-63 cells were investigated.Figure optionsDownload full-size imageDownload as PowerPoint slide