| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233487 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 11 Pages |
•Synthesis of H2PHAT and its metal complexes.•Kinetic studies support first order reaction.•Cd(II), U(VI)O2, Ni(II) and Mn(II) complexes had powerful and complete degradation effect on DNA.
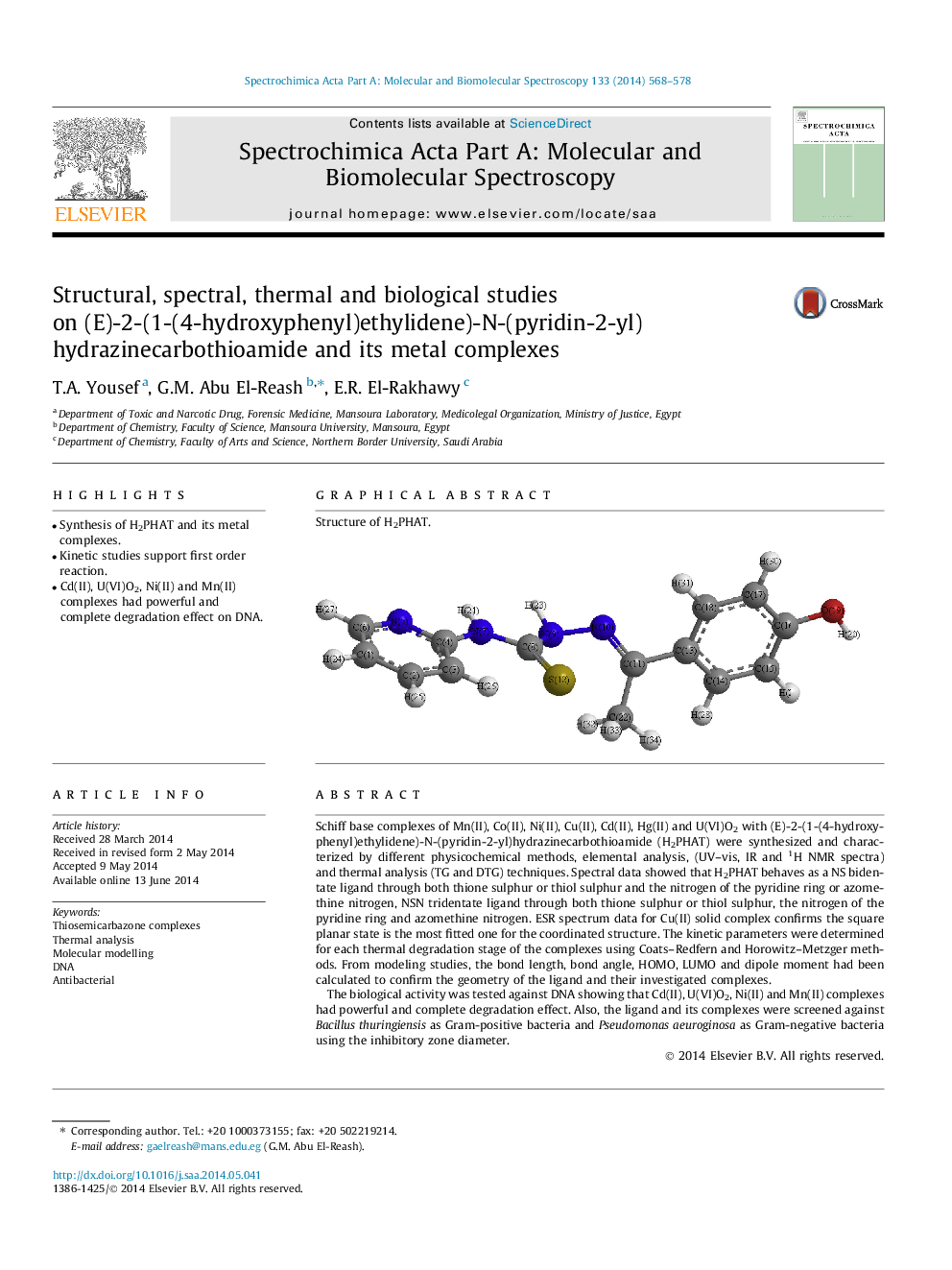
Schiff base complexes of Mn(II), Co(II), Ni(II), Cu(II), Cd(II), Hg(II) and U(VI)O2 with (E)-2-(1-(4-hydroxyphenyl)ethylidene)-N-(pyridin-2-yl)hydrazinecarbothioamide (H2PHAT) were synthesized and characterized by different physicochemical methods, elemental analysis, (UV–vis, IR and 1H NMR spectra) and thermal analysis (TG and DTG) techniques. Spectral data showed that H2PHAT behaves as a NS bidentate ligand through both thione sulphur or thiol sulphur and the nitrogen of the pyridine ring or azomethine nitrogen, NSN tridentate ligand through both thione sulphur or thiol sulphur, the nitrogen of the pyridine ring and azomethine nitrogen. ESR spectrum data for Cu(II) solid complex confirms the square planar state is the most fitted one for the coordinated structure. The kinetic parameters were determined for each thermal degradation stage of the complexes using Coats–Redfern and Horowitz–Metzger methods. From modeling studies, the bond length, bond angle, HOMO, LUMO and dipole moment had been calculated to confirm the geometry of the ligand and their investigated complexes.The biological activity was tested against DNA showing that Cd(II), U(VI)O2, Ni(II) and Mn(II) complexes had powerful and complete degradation effect. Also, the ligand and its complexes were screened against Bacillus thuringiensis as Gram-positive bacteria and Pseudomonas aeuroginosa as Gram-negative bacteria using the inhibitory zone diameter.
Graphical abstractStructure of H2PHAT.Figure optionsDownload full-size imageDownload as PowerPoint slide