| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233493 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 13 Pages |
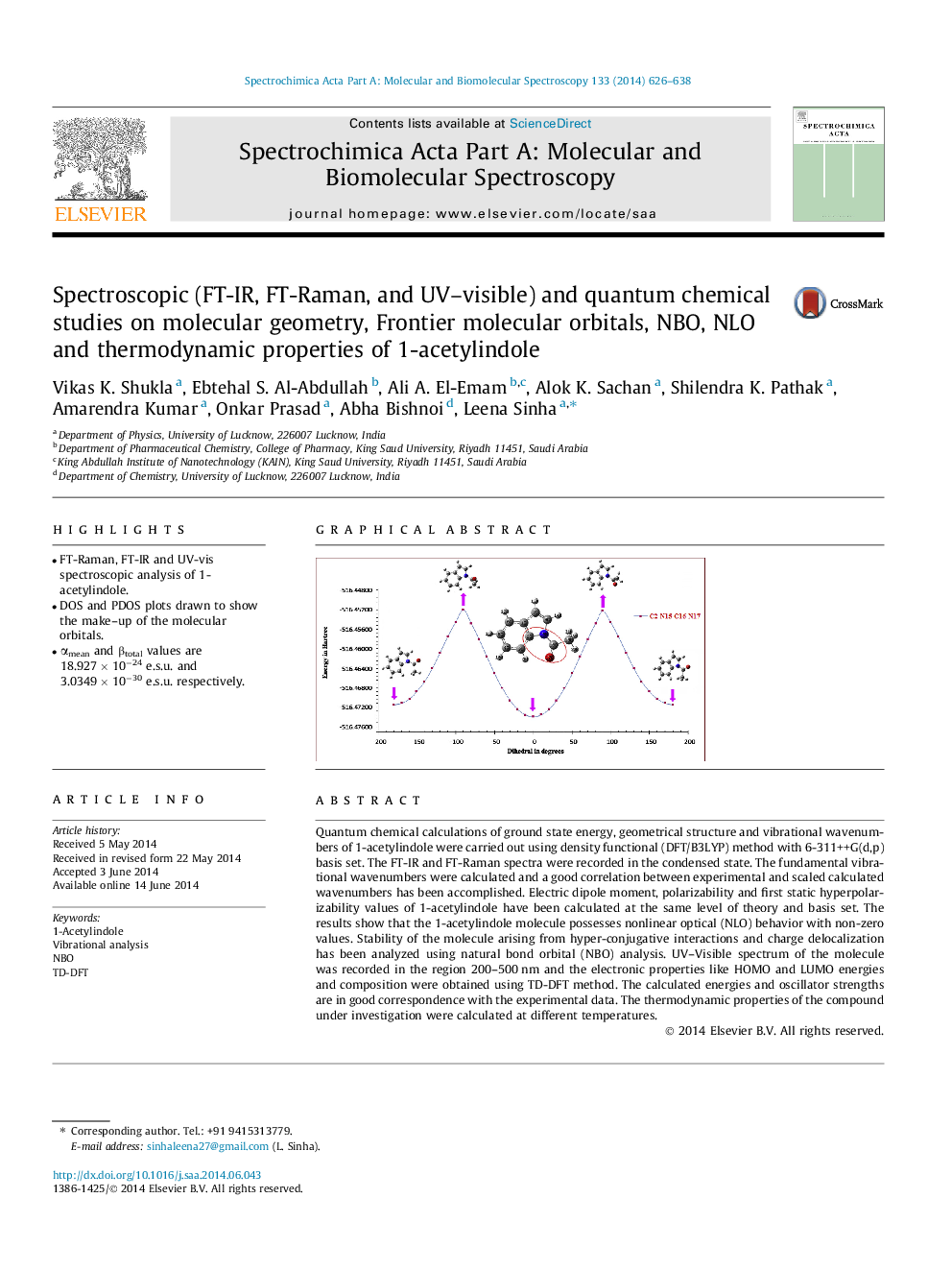
•FT-Raman, FT-IR and UV-vis spectroscopic analysis of 1-acetylindole.•DOS and PDOS plots drawn to show the make–up of the molecular orbitals.•αmean and βtotal values are 18.927 × 10−24 e.s.u. and 3.0349 × 10−30 e.s.u. respectively.
Quantum chemical calculations of ground state energy, geometrical structure and vibrational wavenumbers of 1-acetylindole were carried out using density functional (DFT/B3LYP) method with 6-311++G(d,p) basis set. The FT-IR and FT-Raman spectra were recorded in the condensed state. The fundamental vibrational wavenumbers were calculated and a good correlation between experimental and scaled calculated wavenumbers has been accomplished. Electric dipole moment, polarizability and first static hyperpolarizability values of 1-acetylindole have been calculated at the same level of theory and basis set. The results show that the 1-acetylindole molecule possesses nonlinear optical (NLO) behavior with non-zero values. Stability of the molecule arising from hyper-conjugative interactions and charge delocalization has been analyzed using natural bond orbital (NBO) analysis. UV–Visible spectrum of the molecule was recorded in the region 200–500 nm and the electronic properties like HOMO and LUMO energies and composition were obtained using TD-DFT method. The calculated energies and oscillator strengths are in good correspondence with the experimental data. The thermodynamic properties of the compound under investigation were calculated at different temperatures.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide