| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233498 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 8 Pages |
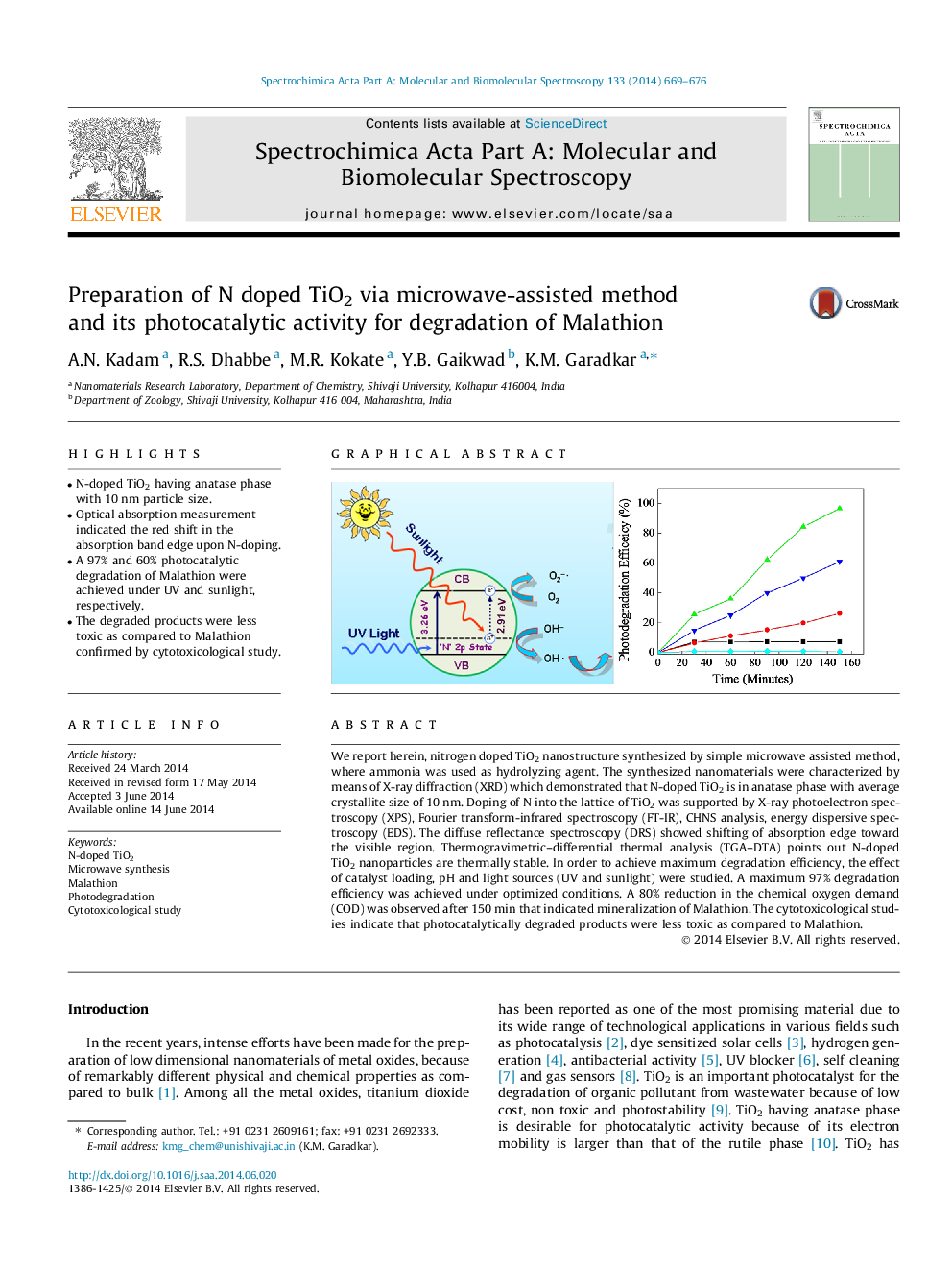
•N-doped TiO2 having anatase phase with 10 nm particle size.•Optical absorption measurement indicated the red shift in the absorption band edge upon N-doping.•A 97% and 60% photocatalytic degradation of Malathion were achieved under UV and sunlight, respectively.•The degraded products were less toxic as compared to Malathion confirmed by cytotoxicological study.
We report herein, nitrogen doped TiO2 nanostructure synthesized by simple microwave assisted method, where ammonia was used as hydrolyzing agent. The synthesized nanomaterials were characterized by means of X-ray diffraction (XRD) which demonstrated that N-doped TiO2 is in anatase phase with average crystallite size of 10 nm. Doping of N into the lattice of TiO2 was supported by X-ray photoelectron spectroscopy (XPS), Fourier transform-infrared spectroscopy (FT-IR), CHNS analysis, energy dispersive spectroscopy (EDS). The diffuse reflectance spectroscopy (DRS) showed shifting of absorption edge toward the visible region. Thermogravimetric–differential thermal analysis (TGA–DTA) points out N-doped TiO2 nanoparticles are thermally stable. In order to achieve maximum degradation efficiency, the effect of catalyst loading, pH and light sources (UV and sunlight) were studied. A maximum 97% degradation efficiency was achieved under optimized conditions. A 80% reduction in the chemical oxygen demand (COD) was observed after 150 min that indicated mineralization of Malathion. The cytotoxicological studies indicate that photocatalytically degraded products were less toxic as compared to Malathion.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide