| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233508 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 7 Pages |
•Imidazole substituted metal phthalocyanine (Im–Pc) complexes were synthesized.•Absorption, fluorescence, triplet state and singlet oxygen formation were studied.•All Im–Pc compounds show high quantum yield of triplet and singlet oxygen formation.•All Im–Pc compounds still keep good fluorescence properties for PDT.
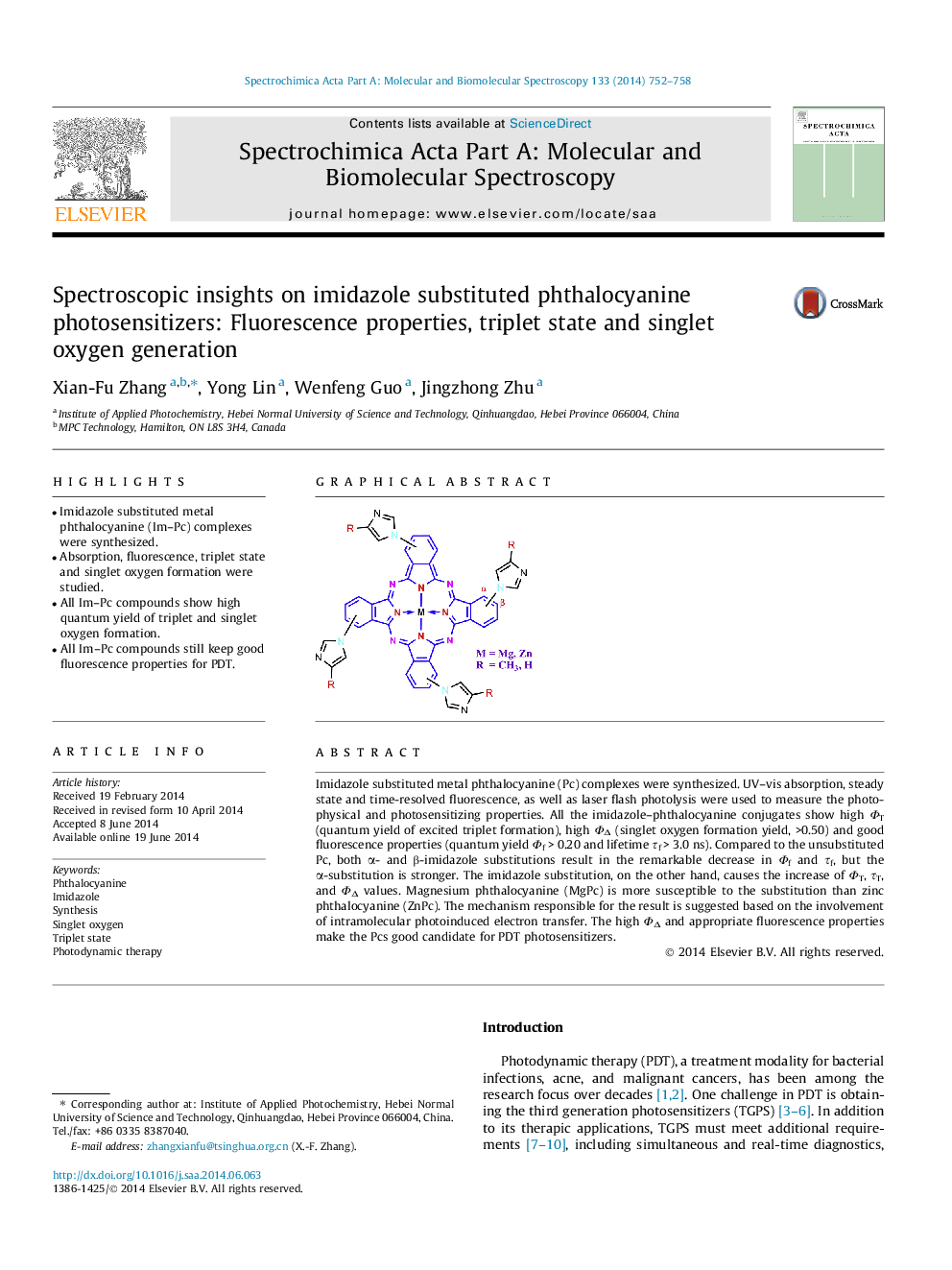
Imidazole substituted metal phthalocyanine (Pc) complexes were synthesized. UV–vis absorption, steady state and time-resolved fluorescence, as well as laser flash photolysis were used to measure the photophysical and photosensitizing properties. All the imidazole–phthalocyanine conjugates show high ΦT (quantum yield of excited triplet formation), high ΦΔ (singlet oxygen formation yield, >0.50) and good fluorescence properties (quantum yield Φf > 0.20 and lifetime τf > 3.0 ns). Compared to the unsubstituted Pc, both α- and β-imidazole substitutions result in the remarkable decrease in Φf and τf, but the α-substitution is stronger. The imidazole substitution, on the other hand, causes the increase of ΦT, τT, and ΦΔ values. Magnesium phthalocyanine (MgPc) is more susceptible to the substitution than zinc phthalocyanine (ZnPc). The mechanism responsible for the result is suggested based on the involvement of intramolecular photoinduced electron transfer. The high ΦΔ and appropriate fluorescence properties make the Pcs good candidate for PDT photosensitizers.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide