| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233510 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 6 Pages |
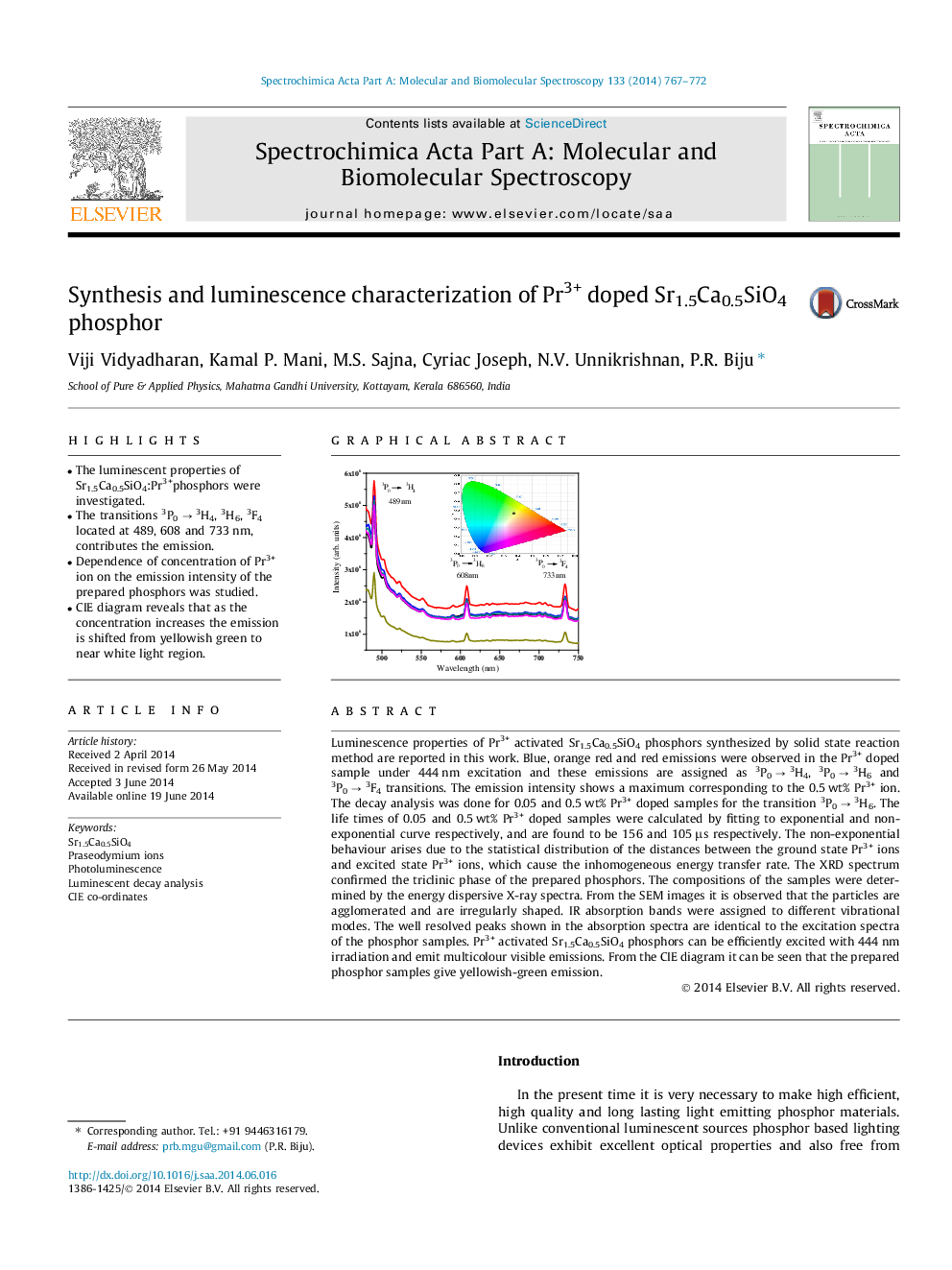
•The luminescent properties of Sr1.5Ca0.5SiO4:Pr3+phosphors were investigated.•The transitions 3P0 → 3H4, 3H6, 3F4 located at 489, 608 and 733 nm, contributes the emission.•Dependence of concentration of Pr3+ ion on the emission intensity of the prepared phosphors was studied.•CIE diagram reveals that as the concentration increases the emission is shifted from yellowish green to near white light region.
Luminescence properties of Pr3+ activated Sr1.5Ca0.5SiO4 phosphors synthesized by solid state reaction method are reported in this work. Blue, orange red and red emissions were observed in the Pr3+ doped sample under 444 nm excitation and these emissions are assigned as 3P0 → 3H4, 3P0 → 3H6 and 3P0 → 3F4 transitions. The emission intensity shows a maximum corresponding to the 0.5 wt% Pr3+ ion. The decay analysis was done for 0.05 and 0.5 wt% Pr3+ doped samples for the transition 3P0 → 3H6. The life times of 0.05 and 0.5 wt% Pr3+ doped samples were calculated by fitting to exponential and non-exponential curve respectively, and are found to be 156 and 105 μs respectively. The non-exponential behaviour arises due to the statistical distribution of the distances between the ground state Pr3+ ions and excited state Pr3+ ions, which cause the inhomogeneous energy transfer rate. The XRD spectrum confirmed the triclinic phase of the prepared phosphors. The compositions of the samples were determined by the energy dispersive X-ray spectra. From the SEM images it is observed that the particles are agglomerated and are irregularly shaped. IR absorption bands were assigned to different vibrational modes. The well resolved peaks shown in the absorption spectra are identical to the excitation spectra of the phosphor samples. Pr3+ activated Sr1.5Ca0.5SiO4 phosphors can be efficiently excited with 444 nm irradiation and emit multicolour visible emissions. From the CIE diagram it can be seen that the prepared phosphor samples give yellowish-green emission.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide