| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1233800 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2013 | 8 Pages |
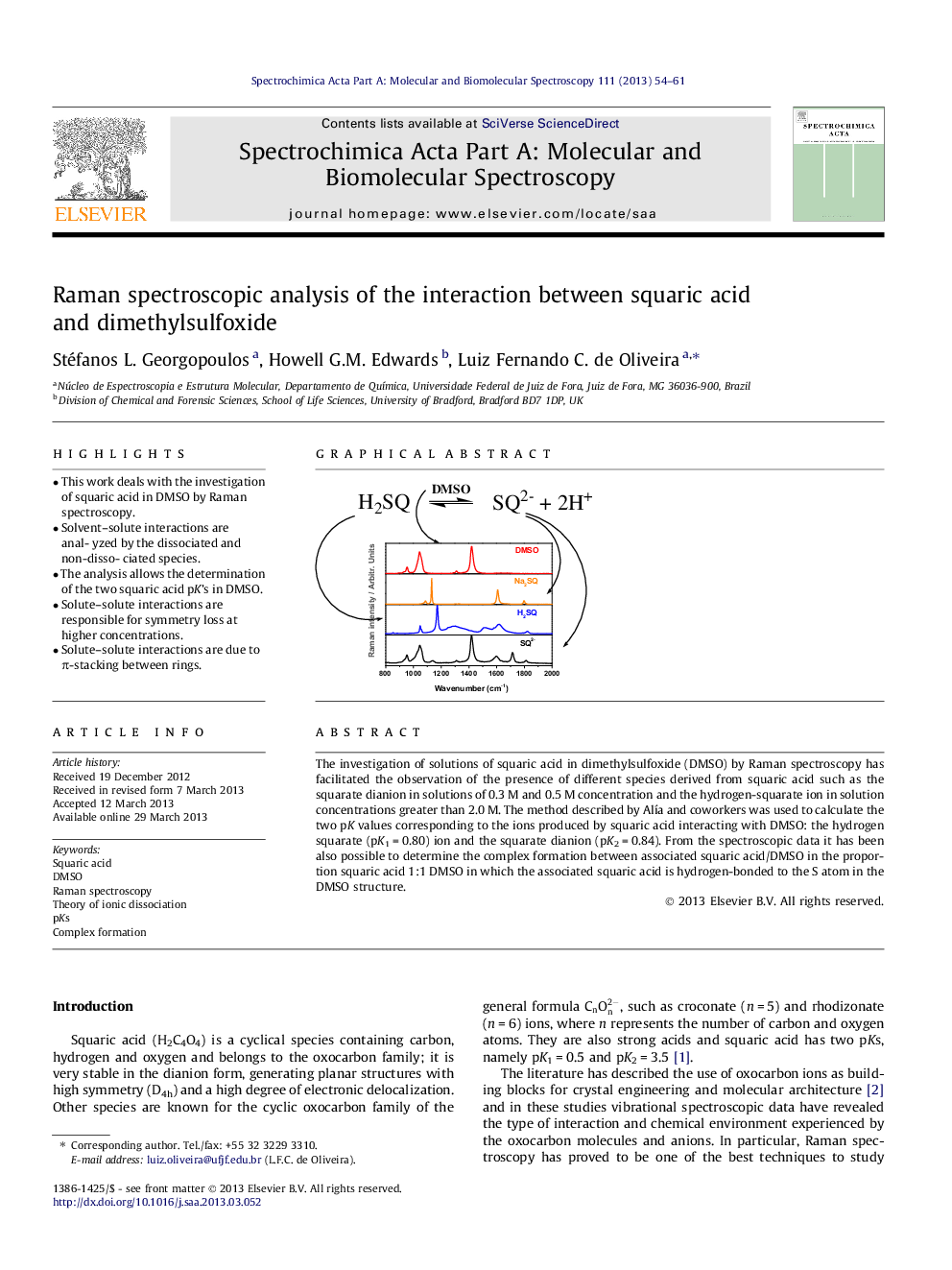
•This work deals with the investigation of squaric acid in DMSO by Raman spectroscopy.•Solvent–solute interactions are analyzed by the dissociated and non-dissociated species.•The analysis allows the determination of the two squaric acid pK’s in DMSO.•Solute–solute interactions are responsible for symmetry loss at higher concentrations.•Solute–solute interactions are due to π-stacking between rings.
The investigation of solutions of squaric acid in dimethylsulfoxide (DMSO) by Raman spectroscopy has facilitated the observation of the presence of different species derived from squaric acid such as the squarate dianion in solutions of 0.3 M and 0.5 M concentration and the hydrogen-squarate ion in solution concentrations greater than 2.0 M. The method described by Alía and coworkers was used to calculate the two pK values corresponding to the ions produced by squaric acid interacting with DMSO: the hydrogen squarate (pK1 = 0.80) ion and the squarate dianion (pK2 = 0.84). From the spectroscopic data it has been also possible to determine the complex formation between associated squaric acid/DMSO in the proportion squaric acid 1:1 DMSO in which the associated squaric acid is hydrogen-bonded to the S atom in the DMSO structure.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide